Covid-19 and the Brain: Innovative Cell Culture Tools to Advance Research
Researchers have begun to dissect SARS-CoV-2 effects on the blood brain barrier, making use of innovative cell culture models and techniques
Patient to patient variability
Its known that the SARS-Cov-2 virus causes variable Covid-19 disease severities on infected patients. The disease can range from mild to acute, from respiratory distress to organ failure. Covid-19 disease has strong correlations to co-morbidities including diabetes, hypertension, obesity, and other health complications.
Ubiquitous expression of the receptor - ACE2
The main receptor for the virus, ACE2, is expressed in nearly every organ system with tissues such as lung and nasal epithelia serving as key entry points into the body. Variability of Covid-19 disease is in part due to variable expression of the ACE2 receptor across body systems and between patients.
SARS-CoV-19 effects on the brain
The brain also appears susceptible to SARS-CoV-2. The loss of smell and taste, strongly associated with active infection, indicates disconnects in sensing tissues and neurological function. Patients with hypertension and dementia, among other ailments, are victim to dizziness, headache, nausea, and impaired consciousness. Active investigations of neurological effects are ongoing.
Crossing the blood brain barrier
A missing area of insight has involved effects the SARS-CoV-2 virus on the central gate-keeper between the circulatory system and the central nervous system -- the Blood-Brain Barrier (BBB). Brain epithelial cells express the ACE2 receptor and, based on knowledge of vascular tissue disruption, it was hypothesized brain epithelia would also be targeted during infection.
Recent Research
In a recent study entitled, “The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier”, researchers used brain epithelial cells and novel brain models to demonstrate direct effects of exposure to the spike protein from the SARS-CoV-2 surface, the mediator of binding with ACE2. Results indicated:
- ACE2 is ubiquitously expressed throughout the frontal cortex vasculature, as detected from post-mortem tissue analysis.
- ACE2 was also detected in primary human brain microvascular endothelial cells, under in vitro cell culture conditions.
- When spike protein and protein components were added to a 2D in vitro model of the blood-brain barrier, significant changes in barrier integrity were observed.
- Using an advanced 3D microfluidic model of the human BBB, a platform that closely resembles the physiological conditions of this CNS intersection, the damaging effects of the spike protein on tissue integrity and function were evident.
The authors concluded that interaction of virus spike proteins with BBB epithelia is thought to trigger a pro-inflammatory response, leading to a cascade of damage and loss of BBB integrity.
Innovative Cell Culture Tools
Beyond providing novel results on the direct impact of SARS-CoV-2 on brain epithelia, the study exemplifies the use of both traditional cell culture techniques and innovative cell culture models to approach challenging questions.
2D Cell Culture

Primary human brain microvascular endothelial cells (hBMVECs) used were isolated from healthy tissue and subjected to culture conditions which included: rat tail collagen I coated flasks and full growth medium supplemented with EGM-2 microvascular endothelial SingleQuots Kit (Lonza). In certain experiments, an immortalized cell line (hCME/D3) often used for modeling the BBB was used.
- Cytoxicity experiments were performed to evaluate the toxicity of SARS-CoV-2 spike protein on hBMVEC cells. Data was acquired by measuring excitation and emission of DNA-sensitive dyes used a UV-Vis microplate reader (SpectraMax M5e shown right).
- Cell permeability assays were performed using confluent monolayers of cells incubated in the basolateral partition of a growth incubation chamber. SARS-Cov-2 spike protein, spike protein components, and controls were applied to the cells and incubated. Dye-conjugated dextran was then added to the apical chamber, and cell permeability was determined by fluorescent measurements of the basolateral compartment, versus control untreated cells, using a fluorescence plate reader.
- Additional experiments were performed including, flow cytometry, RT-PCR, Western blot, and others, in order to characterize the cells and proteins essential to the cell culture assays.
3D Blood Brain Barrier Model
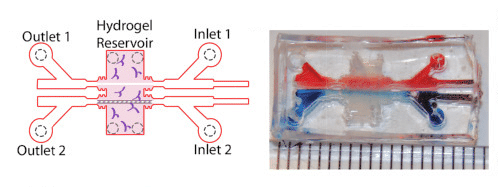
The study also took advantage of advanced cell culture platform, including a three-dimensional model of the blood brain barrier, fabricated by polymerized hydrogels composed of type I collagen, hyaluronan, and Matrigel within a micro-fabricated device.
- Two parallel, cylindrical voids were created in the gel, within which hCMEC/D3 cells (BBB mimicking) were coated and incubated to promote cell attachment.
- Following seeding, cells were subjected to shear stress using a linear syringe pump to establish barrier function.
- Those cells were then subjected to incubation with various SARS-CoV-2 spike protein components, and gel vessels were either placed in fixative or prepared for permeability experiments.
- Fluorescence microscopy imaging (right) allowed determination of localization of virus protein to the cell-cell junctions of fixed cells.
- In cell permeability assays, 3D vessels were transferred to the stage of an inverted epifluorescence microscope enclosed by an environmental chamber set to 37° C, 5% CO2, and 95% humidity.
The 2D cell culture model made use of more standard techniques for incubation and seeding of cell monolayers, whereas the 3D BBB model microfluidic device used in the study created the context for delicate cell stress and permeability assays using tissue-like conditions.
Access to high-quality cell culture media and components is an absolute requirement to ensure experimental models, like those described, can function as intended and provide definitive results. Furthermore, cell culture models like those described require proven instrumentation and advanced technology in order to produce actionable results from challenging assays.
Summary
The synergy between standard techniques and advanced cell culture models is enabling cutting edge research at ever-increasing depths of insight and discovery. The value of this synergy is clearly apparent as we leverage all available tools in the fight against the novel coronavirus.
Image of 3D hydrogel BBB model adapted from:
Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier
View microplate readers and microscope listings on LabX.com.