Polymerase Chain Reaction (PCR): Tips and Technologies
Pioneered in 1980 by Kary Mullis, polymerase chain reaction (PCR) has proven to be a revolutionary and essential tool in modern molecular biology. The technology has brought molecular genetics and cloning to everyday life science labs, providing a ready means to create constructs, clone genes, and manipulate cells in a multitude of ways.
PCR today encompasses a range of technologies and approaches making the concept more empowering than ever. Here we review popular technologies and the equipment involved, briefly discussing important aspects to keep in mind when optimizing the technique for your needs.
Takeaways
1. Determine the desired product or application – cloning, sequencing, quantitative expression analysis, reverse transcription expression analysis, etc.
2. Identify the source and type of DNA – plasmid or chromosome, unpurified cell lysate, cell-free DNA from biofluids, etc.
3. Implement the appropriate PCR method suited for the above application – traditional PCR, nested PCR, qPCR, rtPCR, etc.
4. Modify the PCR components to produce the optimal fidelity, processivity, and yield -- polymerase, buffer mix, melting temperature, annealing temperatures, thermocycle protocol, etc.
5. Test the PCR products to ensure reliability and quality control -- restriction enzyme digests, gel analysis, cloning screens, etc.
6. Locate a source with the best options for PCR/Thermocycler and real-time PCR equipment, associated reagents, kits, and supplies.
PCR Procedure
As a review, the PCR concept is based on using DNA polymerase to synthesize new strands of DNA complementary to the template DNA that is provided. As DNA polymerase can only add nucleotides to the 3’ OH end group, the enzyme requires a primer (small segment of DNA) to anneal (bind) to the template in order to start the process of elongation. The primer sequence allows the technique to be directed to (theoretically) any region of the DNA template, thus making PCR both specific and cumulative – capable of producing billions of copies (amplicons) from a single DNA template.
PCR Steps
Denaturation – At the beginning of the PCR reaction, the DNA is subjected to high temperature in order to separate (melt) double stranded DNA into its single stranded components.
Annealing – Upon single-strand formation, the temperature is then adjusted to an ideal point specific for binding of the primer to its complementary template sequence.
Extension – Once annealed, the primer is extended by the action of DNA polymerase and 3’ OH addition of nucleotides complementary to their template partners.
The steps repeat during the course of a typical reaction in a process called thermocycling – hence the term thermocycler used for the equipment involved.
DNA Polymerase
Since PCR involves high temperature cycling, a compatible thermostable DNA polymerase such as Taq polymerase (derived from Thermus aquaticus) is employed. An inherent challenge in PCR is non-specific activity of the polymerase during set up and initiation of the reaction. For this reason, many techniques have been used including preparation of components on ice and initiation of the melting stage prior to DNA polymerase addition (hot start), among others. An issue with Taq polymerase is that its half-life of activity diminishes significantly over 90 °C, necessitating additional supplements during traditional cycling and limiting its utility in some PCR applications.
To address this, Pfu DNA polymerase (Pyrococcus furiosus) was introduced with 20 x greater stability at 95 °C. Subsequently, additional thermophilic enzymes were brought into the mix with similar robust properties – although inherent problems with these natural enzymes have included slow DNA synthesis (or processivity), inabilities to amplify uracil-containing DNA, and other limitations.
Fidelity, or the accuracy of synthesis, is another critically important measure of the polymerase. It involves not only thermostability but the proofreading activities of the DNA polymerase as well -- crucial to error-free DNA and applications such as cloning, sequencing, and site-directed mutagenesis. We won’t go into the mechanisms or tests of proofreading activities here, we’ll only mention that Pfu possess fidelities 10 x that of Taq polymerase – an essential consideration when weighing accuracy versus processivity for a particular application.
Modern “next generation” high-fidelity DNA polymerases are engineered with fidelities >50-100 x that of Taq. Examples include Platinum SuperFi and Q5 Hot Start High-Fidelity from ThermoFisher, designed to offer performance in High-throughput PCR, Long PCR, and difficult GC-rich template PCR, in additional to traditional applications. We will skip over detailed analysis of the types and the range of DNA templates for now, and move on to PCR methods for various downstream applications.
PCR Methods
Conventional PCR
As briefly touched upon, traditional PCR involves use of a primer or set of primers to elongate DNA synthesis prior to purification and analysis for downstream use. The melting temperature of the template DNA must be considered along with the primer-template melting point in order to balance fidelity with processivity. Use of a Tm calculator can help in the design of primers and modification of the conditions in order to increase the yield of the reaction.
Hot Start PCR and Touchdown PCR can be employed to minimize non-specific reactions – essential particularly at the initial stage to prevent subsequent multiplication and substantial contamination of the product. In Touchdown PCR, higher temperatures are used initially to destabilize formation of primer-dimers and non-specific primer-template complexes, thereby minimizing early contamination of the reaction. The temperature is subsequently lowered to increase processivity and yield, now that exponential duplication has begun.
Nested PCR
Conventional PCR typically makes use of primers designed with internal restriction enzyme cleavage sites. In subsequent processing, testing, and cloning, these sites provide a means to semi-quantitatively measure yield and manipulate the product.
Nested PCR is a modification that enhances the specificity and yield of the PCR product. The technique makes use of an initial set of primers, and a nested set of primers, for amplification of precise regions of DNA. By first enriching for the region of interest using the initial primers, the purity of the template has been increased dramatically – greatly influencing the fidelity, processivity, and yield of subsequent cycles using the nested primers.
Direct PCR – Fast PCR- GC-rich PCR – Multiplex PCR – Long PCR
All of these approaches leave room for optimization of enzymes, buffers, and cycling conditions in order to accommodate DNA from various sources, of variable GC-content and complexity, and for variable throughputs.
The methods above can be performed using standard thermocycler equipment with the ability to create and store custom cycling methods to match each application.
Quantitative (qPCR)
This PCR method builds off the limitations of end-point analysis for accurate measurement of reaction product concentrations. Traditional PCR proceeds through a series of growth phases in DNA copy amount. Following initiation, the exponential or log growth phase proceeds until either the primer, the polymerase, or the cofactors are depleted – at which time the reaction slows down to the linear phase, followed inevitably by the plateau phase.
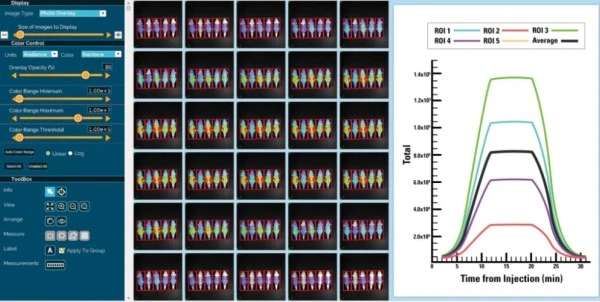
Quantitative PCR (also known as real-time PCR) makes use of real-time monitoring of fluorescence intensity associated with DNA amplification during the exponential phase. This method greatly increases the accuracy of DNA measurement compared to end-point analysis, with limitations now dependent simply on the resolving power of fluorescence detection.
High quality detection is the basis for specialized instrumentation especially designed for qPCR, with customizable features to suit a range of desired applications. Other quantitative methods, such as digital or limiting dilution PCR, make use of chip-based partitioning of individual reactions – requiring additional equipment and analysis tools.
PCR Applications
There is near boundless potential for PCR, for instance in food and beverage testing, pharmaceutical research and development, toxicology and forensics, and a number of additional industries. Various PCR methods are and have been in use for some time in these areas. In order the see widespread use in clinical diagnostics settings such as field-based or point-of care facilities, several aspects are in need of refinement -- a major one being speed. Automation, microfluidics, and micro processing are evolving to address the speed issue.
Despite the challenges, future advancements may make PCR both powerful and indispensable for nearly all facets of laboratory and clinical use.
View PCR and Thermocycler Equipment Listings at LabX.com
View Real-Time QPCR Equipment Listings at LabX.com
View Reagents and Synthesis Listings at LabX.com
View PCR Enclosures and Accessories at LabX.com
Updated February 2022