LabX Reasons to Upgrade: The BioTek 50 TS Microplate Washer
Modern updates to a trusted performer
View the BioTek 50 TS and request a quote on LabX.com
View and download the pdf version of this article
BioTek is an industry leader in life science instrumentation, including liquid handling, multi-mode microplate detection, imaging and microscopy and automation designed to serve a wide range of applications. BioTek products enable research by providing high-performance, cost-effective solutions to challenging tasks in many application workflows.
The original BioTek ELx50 microplate washer line was built with these qualities in mind, serving as a workhorse since it’s release in 1997.
The new upgrade to this line, the 2017 BioTek 50 TS, adds significant enhancements in terms of throughput, versatility, and user control, resulting in dramatic improvements to an already proven platform.
BioTek’s suite of liquid handling devices include microplate washers, combined washer/ dispensers, reagent dispensers, and automated pipetting systems. The ELx50 was designed to suit a range of applications from basic ELISA to sensitive cell- and bead-washing (including Luminex xMAP technology). The ELx50 was built on a flexible platform that enabled a range of assay formats owing to it’s modular design capabilities.
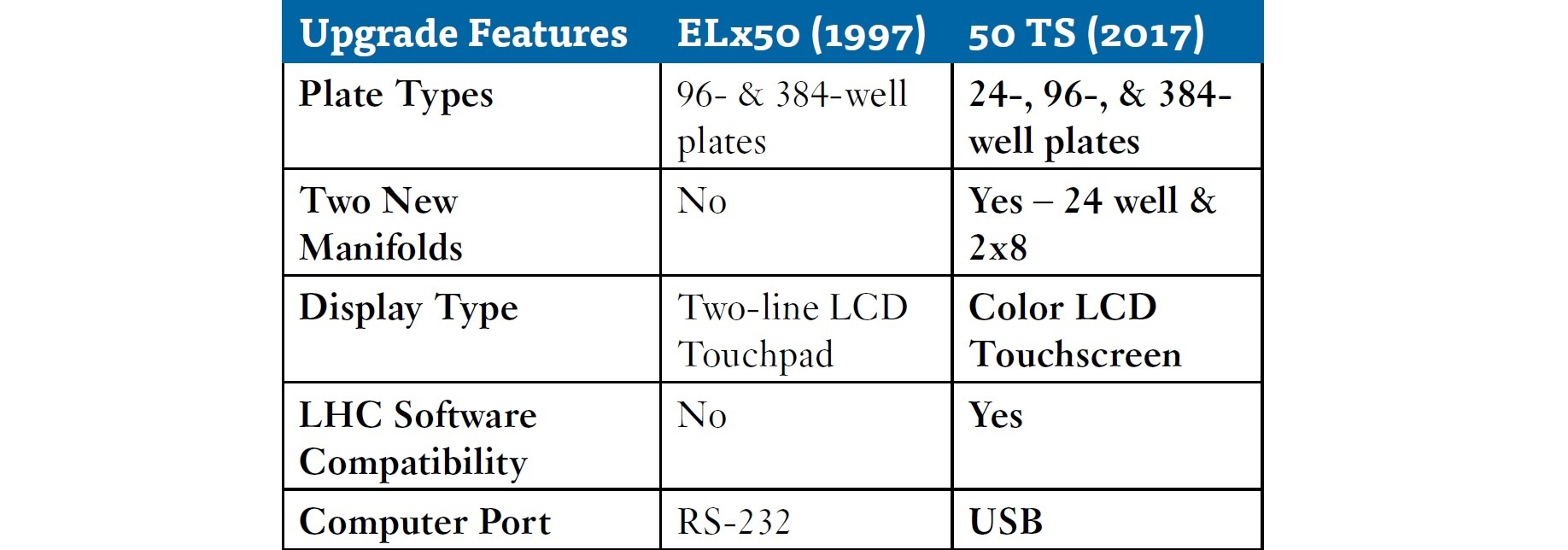
The new BioTek 50 TS upgrades over this platform include: expanded plate compatibility, two new plate manifolds, a new color touchscreen interface, and PC software compatibility.
The two new manifold options allow greater diversity in plate type, processing speed, and experimental format.
- The 50 TS line can accommodate 24-well plates in addition to traditional 96- and 384-well plates seen with the older model.
- The new optional 24-well plate manifold changes the throughput by allowing larger volume cell-based assays to be processed, while at the same time expanding versatility to accommodate a greater range of cell or tissue types and experimental formats.
The 50 TS’s manifolds allow optimized control and positioning of both the aspirate and dispense sections of the wash manifold. The benefits include rapid washing of both standard and sensitive cell-based assay plates (which require gentle handling) as well as high accuracy fluid level control.
- The new 2x8 manifold option also increases processing rates of 96 well plates over previous ELx50 models.
- The difference in speed is significant: 50 TS, 96-well, 2x8-tube, 300µL/well, <80 sec; compared with ELx50, 96-well, 8-tube manifold, 300µL/well, <130 sec.
Improvements include the user interface and connectivity features. The 50 TS display includes a user-friendly color LCD touchscreen, a stark comparison to the two-line LCD touchpad used previously. The computer ports have changed as well from a single RS-232 to 3 USB ports for computer control by LHC software, for which the 50 TS is fully compatible.
- The LHC software and LCD touchscreen allow up to 75 unique stored protocols to be run directly from the main menu.
- The Protocol option enables users to program aspirate, dispense, and wash steps and specify parameters such as volume, rates, cycles, etc.
- The pre-configured Quick Prime and Wash methods allow users to perform any function outside of a programmed protocol.
- The Maintenance method permits pre-programmed maintenance and quality control protocols to be executed, while the Instrument method controls instrument configurations and user access.
Updated LHC v2.20 software permits smooth transfer of protocols between the computer and the instrument.
- A lock feature prevents unwanted onboard editing of files, while a new prompt alert makes users aware when advancing through steps of a protocol.
- A software-controlled utility tool allows users to optimize tip placement within each well. The 50 TS instruments have a modern and versatile design
- The instrument housing includes an esthetic and ergonomically improved cover.
- A host of additional accessories are available as well including the Level Alert System, buffer bottle and supply tubing configurations, as well as interchangeable manifolds with large aspiration tubes.
In addition to washing, optional modules include vacuum filtration or biomagnetic bead separation.
Ten different configurations are available depending on the preferences for specific plate manifolds, vacuum or separation modules, or automated buffer switching options. The devices are CE and TUV marked, are ROHS compliant, and IVD models are available. Many of the aforementioned modules and options are model specific, with nine versions available for custom applications.
It has been some time since the release of the original ELx50 line and an upgrade to modern technology is certainly welcome. It was undoubtedly a challenge to improve upon a platform that has seen such widespread usage and success. BioTek has done it nonetheless and, with impressive enhancements in versatility, speed, and user control, the 50 TS line is sure to be a strong performer for years to come.
This article was written by LabX and published in conjunction with BioTek.
BioTek Instruments, Inc., headquartered in Winooski, VT, USA, is worldwide leader in the design, manufacture, and distribution of innovative life science instrumentation.
We are the only life science instrumentation company with corporate headquarters, manufacturing, research and development, applications and service in the USA. Our company-wide commitment to quality and value is backed by our superior customer care, technical service centers, scientific application experts and a knowledgeable field sales and service specialists. Our expertise, quest for innovation, and efficiency all combine to provide the best possible technologies for our customers.
BioTek is ISO9001/ISO13485 certified, is an FDA Registered Medical Device Manufacturer, and has appropriate products in compliance with the EU In Vitro Diagnostic Directive (IVDD). Our quality program extends to your laboratory as well. BioTek offers optional validation (IQ/OQ/PQ) and FDA 21 CFR Part 11 tools to ensure regulatory compliance.










