Challenging Oligonucleotide Purifications and the Advantages of Novel HPLC Column Technologies
Approaches such as polymeric reversed-phase separations are seeing expanded use across a growing number of applications
Oligonucleotides (Oligos) are gaining unprecedented popularity for their expanding use in research, therapeutics, and novel vaccine development. Many recent advances including oligonucleotide-based SARS-Cov-2 vaccines have fueled the need for efficient, high-quality methods for oligo production and purification. Conventional approaches towards oligo purification are challenged by a range of limitations, making the preparation of long oligos and derivatized products difficult at the purity and yield necessary for downstream applications.
 Polymeric reversed-phase supports are novel HPLC stationary phases that provide high-efficiency performance over an extended column life in nearly any mobile phase or pH. We previously explored Hamilton’s polymeric reversed-phase (PRP-C18) column
technology and its improved capabilities for long oligo purification as compared
to traditional reversed-phase separations.
Polymeric reversed-phase supports are novel HPLC stationary phases that provide high-efficiency performance over an extended column life in nearly any mobile phase or pH. We previously explored Hamilton’s polymeric reversed-phase (PRP-C18) column
technology and its improved capabilities for long oligo purification as compared
to traditional reversed-phase separations.
Since our initial look, the PRP technology has seen significant adoption and has been implemented in numerous applications ranging from agriculture and food science to cannabis, forensics, and more. Considering the hot topic of oligonucleotide purification and expanding use of PRP technology, we thought it was the perfect time to catch up with Adam Moore Ph.D., HPLC Applications Chemist at Hamilton, to learn more about PRP, the underlying technical details, and its advantages for DNA and RNA purification.
Damon Anderson (LabX) - What characteristics of oligonucleotide therapeutics such as short interfering RNA (siRNA), antisense oligos (ASO), microRNA (mRNA), and RNA vaccines can make it challenging to achieve the required purity and yield using conventional methods?
AM - Purification of Oligonucleotides from their synthesis products, failure sequences, protecting groups, and other starting materials, add to the challenge of purification. The separation of the desired product from the N-1 failure sequence can be problematic when the length of the oligonucleotide sequence is > 20 base pairs. Secondary structures produced by the interaction of complimentary base pairs with its own strand or with neighboring strands, (duplexes, hairpin loops, and guanine quartets) add additional pitfalls.
DA - How do PRP-C18 column separations overcome these challenges?
AM - The really great thing about the PRP-C18 resin is that it has all the base characteristics of a PSDVB polymeric resin, which is known to exhibit good resolution of oligonucleotides, with the bonus of non-polar C18 groups covalently bonded for better resolution amongst similar molecules. The resin is highly tolerant of alkaline mobile phase conditions and can accommodate high buffer conditions and is impervious to elevated column temperatures. The phase will not change or degrade since the octadecane moieties are chemically bonded to the aromatic rings of the divinylbenzene core of the bead.
DA - The preferred method of oligo preparation involves the use of ion-pairing reagents and reversed-phase ion-pairing (RPIP) chromatography to separate oligos based on small, single-base pair differences. What are some of the drawbacks of using conventional RPIP columns?
AM - Some downsides as we have already kind of talked about revolve around the minimization of secondary interactions. In order to overcome those interactions people use column oven temperatures in excess of 60 °C. The second is through the use of a highly alkaline mobile phase, generally, greater than a pH of 12. Unfortunately, most RP media is silica based which degrades under those types of mobile phase conditions.
DA - How do PRP-C18 columns address these challenges?
AM - The PRP-C18 stationary phase easily conforms to these conditions and doesn’t miss a beat. Since there are no silanols in the resin to phase strip, column degradation is held at bay. Additionally, the crosslinked PSDVB core and covalently bonded octadecane units can easily handle over 100 °C and over 1M NaOH mobile phase conditions, while still maintaining good peak efficiency before, after, and during alkaline washing. (Just in case anything gets stuck on the column). Likewise, due to the added hydrophobicity associated with the addition of long non-polar units bonded to non-polar aromatics generating a highly hydrophobic stationary phase, increased retention is observed.
DA - Have you tested PRP-C18 column separations for the ability to handle ion-pairing reagents and other challenges associated with small, single-nucleotide separations?
AM - Yes, we
have. We tested some of the more common
ion-pairing reagents, TEAA, HAA, TBAA, and TBAP. We were looking to see if there was any kind
of trend with alkyl length on the amine salt versus retention. We found that increasing both the number of alkyl
moieties and the length of alkyl groups on the amine led to increased retention.
However, it also led to a decrease in peak efficiency as the steric/electronic effects
of the tetra alkyl groups contributed to reduced mass transfer of the oligonucleotide
analytes with the stationary phase. Temperature
also had an effect on the separation of a 25-mer containing a mixture of N-1,
and N-2 failure sequences; the separation was monitored at 50, 60, and 85 °C,
and the data indicates decreased retention as temperature increased but
provided increased peak efficiency between the highly similar analytes.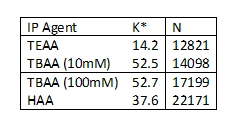
K* - indicates retention
N – Indicates theoretical plate height as a means of peak efficiency or mass transfer.
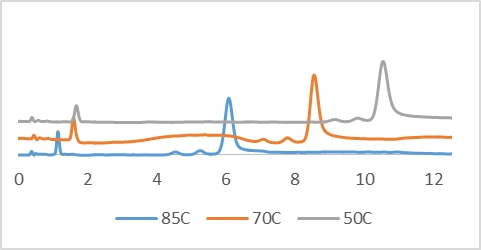
Retention time variance as temperature is varied. FLP, N-1, and N-2 separations shown.
DA - Are these PRP-C18 methods compatible with downstream analysis via mass spectrometry and other techniques used for verification and validation?
AM - Totally. However, the tetrabutyl ammonium phosphate salts are less mass spectroscopy friendly than the tetraalkyl acetate salts.
DA - As a final thought, what do you feel are the most important attributes of PRP technology that will continue to provide advantages for challenging separations and emerging applications?
AM - I think the one thing that maybe we haven’t touched on here is the scale-up attributes that are associated with the PRP-C18 resin. Since it is a porous polymer bead, the loading capacity is dramatically increased compared to core shell or superficially porous particles highlighted in other chromatography resins. The PRP-C18 provides great resolution and ~20% more loading capacity which offers enhanced value when it’s time to transfer from analytical to preparatory isolations. In addition to the loading capacity, the resin exhibits excellent temperature and chemical stability for any type of analyte separation. Even if the failure sequences, starting material, or synthesis protecting groups start to reduce peak efficiency, a harsh hydroxide wash will regenerate the resin back to its original state for more use.
This editorial was written by LabX and published in partnership with Hamilton Company










