The Benefits of Direct Mercury Analysis: A Technology Review
By Robert Thomas
The analytical challenges presented by measuring mercury using techniques like cold vapor atomic absorption/ atomic fluorescence (CVAA/ AF), or Inductively Coupled Plasma- Mass Spectrometry (ICP-MS), are well recognized. The inherent problem lies in the fact that all these techniques are solution based, which means that if the sample is not a liquid it has to be digested before it is introduced to the instrument.
Because of the high volatility of mercury, this can lead to loss of analyte and poor recoveries if the sample is not digested correctly. In addition, even if the sample is a liquid, mercury is notorious for its memory effects, particularly using techniques such as ICP-OES or ICP-MS because it can stick to the walls of the sample container as well as surfaces of the sample introduction system.
To compensate for this, long washout times are typically required to ensure the previous sample is not still resident in the sample line. In some cases, it requires the addition of a few ppm of gold, which acts as a strong oxidizing agent to convert the mercury to the mercuric ion to stop it from being reduced to elemental mercury and lost to the atmosphere or absorbed into the sample line. This is further compounded if the solutions are left for extended periods of time before they are analyzed. The bottom line is when carrying out mercury measurements at low levels, great care must be taken to ensure the data generated is representative of the mercury in the original sample.
Direct Mercury Analysis
One of the most widely accepted ways around these limitations is to carry out the measurement by direct mercury analysis, which is a technique used for the determination of total mercury directly in solid, liquid and gas samples, using the principle of thermal decomposition, amalgamation and atomic absorption.
In this approach, a decomposition furnace is used to release mercury vapor instead of the chemical reduction step used in traditional liquid-based analyzers. Both solid and liquid matrices can be loaded onto the instrument’s autosampler and analyzed without acid digestion or sample preparation prior to analysis. Because this approach does not require the conversion of mercury to mercuric ions, lengthy sample pretreatment steps are unnecessary. As a result, there is no need for reagents such as highly corrosive acids, strong oxidizing agents or reducing chemicals, which means no hazardous waste to be disposed of.
Direct mercury analysis is a well-established analytical technique used by the environmental, biological, clinical, food, industrial and academic communities that has been approved by testing/standards organizations such as the EPA in method 7473 (1) and the ASTM using Method D6722 (2).
Principle of Operation
Direct mercury analysis can be broken down into four distinct steps:
- Thermal decomposition
- Catalytic conversion
- Amalgamation
- Atomic absorption detection
Controlled heating stages are implemented to first dry and then thermally decompose a sample introduced into a quartz tube. A continuous flow of air or oxygen carries the decomposition products through a hot catalyst bed where halogens, nitrogen, and sulfur oxides are trapped, allowing for the analysis of even reactive or flammable samples. All mercury species are reduced to elemental mercury (Hg0) and are then carried along with reaction gases to a gold amalgamator where the mercury is selectively trapped. All non-mercury vapors and decomposition products are flushed from the system by the continuous flow of gas.
The amalgamator is subsequently heated and releases all trapped mercury to the single- or double-beam (depending on model), fixed wavelength atomic absorption spectrophotometer. Absorbance is measured at 253.7 nm as a function of mercury content. Since mercury is thermally released from the sample and preaccumulated, measurements are matrixindependent and instrument calibrations are long-lasting. A schematic of a direct mercury analyzer (DMA-80 evo- Milestone Inc, Shelton, CT) is shown in Figure 1.
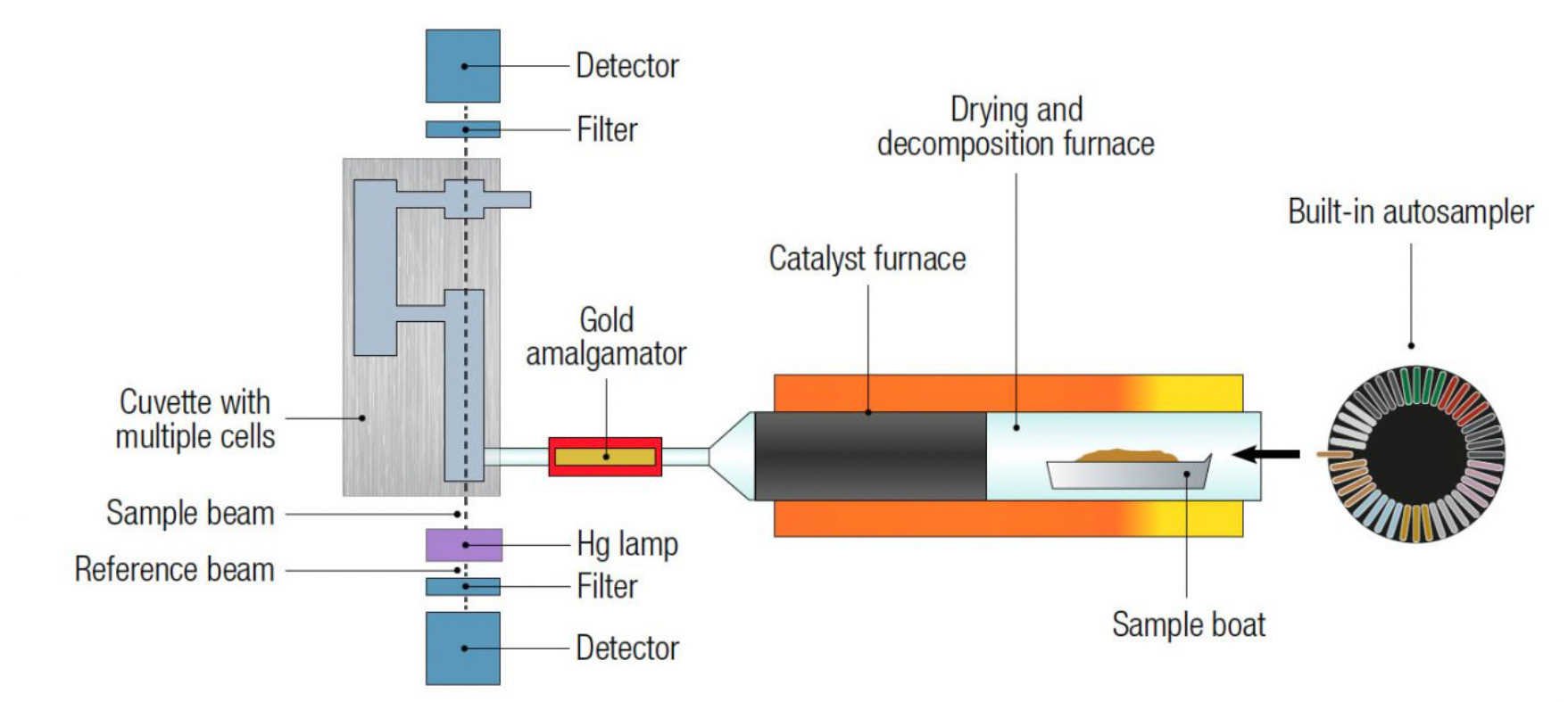
Figure 1: A schematic of the DMA-80 evo direct mercury analyzer
Double-Beam Technology Direct Mercury Analysis: The new DMA-80 evo
The DMA-80 was initially developed using single-beam AA technology. In this technique, a beam of light (photons) from the hollow cathode light source is directed through the sample compartment, where the analyte atoms generated by the excitation source absorb a characteristic wavelength of light, which is then passed into the optical system, detected and converted into an electrical signal.
Since variations in the lamp energy can potentially compromise precision and detection capability, for the very first time, the benefits of double-beam technology have now been incorporated into the design of a direct mercury analyzer with the Milestone DMA-80 evo. With this technology, a reference beam monitors the lamp energy whereas the sample beam reflects absorption of the analyte photons. The observed absorbance measurement is the ratio of the sample and reference beams.
Doublebeam technology compensates for the effects due to drift in lamp intensity, electronic and mechanical fluctuations, and thermal instability that affect both the sample and reference beams equally. This significantly increases the signalto- noise ratio, which greatly improves the stability of the signal, resulting in a lower limit of quantification and enabling more accurate and precise mercury determinations even at the low-ppt level.
Auto Blank
In addition to the unique double-beam technology, the DMA-80 evo also incorporates software which recognizes the mercury level of a sample and, in the case of high concentrations, introduces blank cycles in the sample run to clean the system prior to running the next one. After this cycle, the instrument evaluates the blank level and proceeds automatically to the next sample.
For example, if the Hg level is above 10 ng as previously determined by the user, the software will start blank cycles to clean the system prior to processing the next sample. Then, the blank cycles are carried out until the Hg level is below 0.5 ng (using a maximum of 2 blank cycles). At this point, the analysis will continue. If the blank cycle value is still over 0.5 ng, the operator can abort at any time and the Auto Blank feature will stop. Both the mercury limits and the amount of blank cycles can be modified.
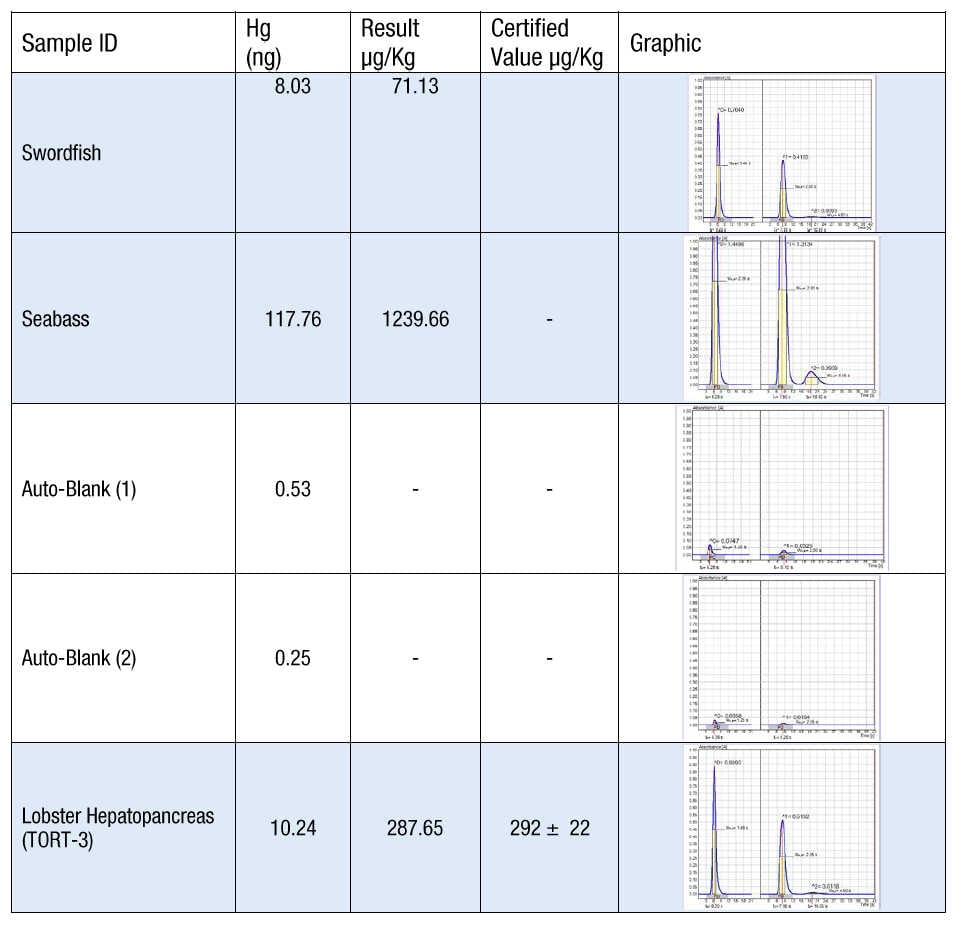
Figure 2: Data showing the DMA-80 evo Auto Blank feature
This process is demonstrated in Figure 2, which shows the measurement of two fish samples (swordfish and sea bass) of 8 and 118 ng, respectively, that are automatically followed by two auto blanks until the desired blank value of 0.5 ng is reached. A TORT 3 lobster CRM is analyzed at the end of the run, showing good agreement with the certified value of 288 μg/kg. The atomic absorption peak profiles of samples, blanks and CRM are shown in the far right hand column for confirmation purposes. This Auto Blank feature, which can be customized based on mercury levels, therefore allows for the highest accuracy particularly at trace levels.
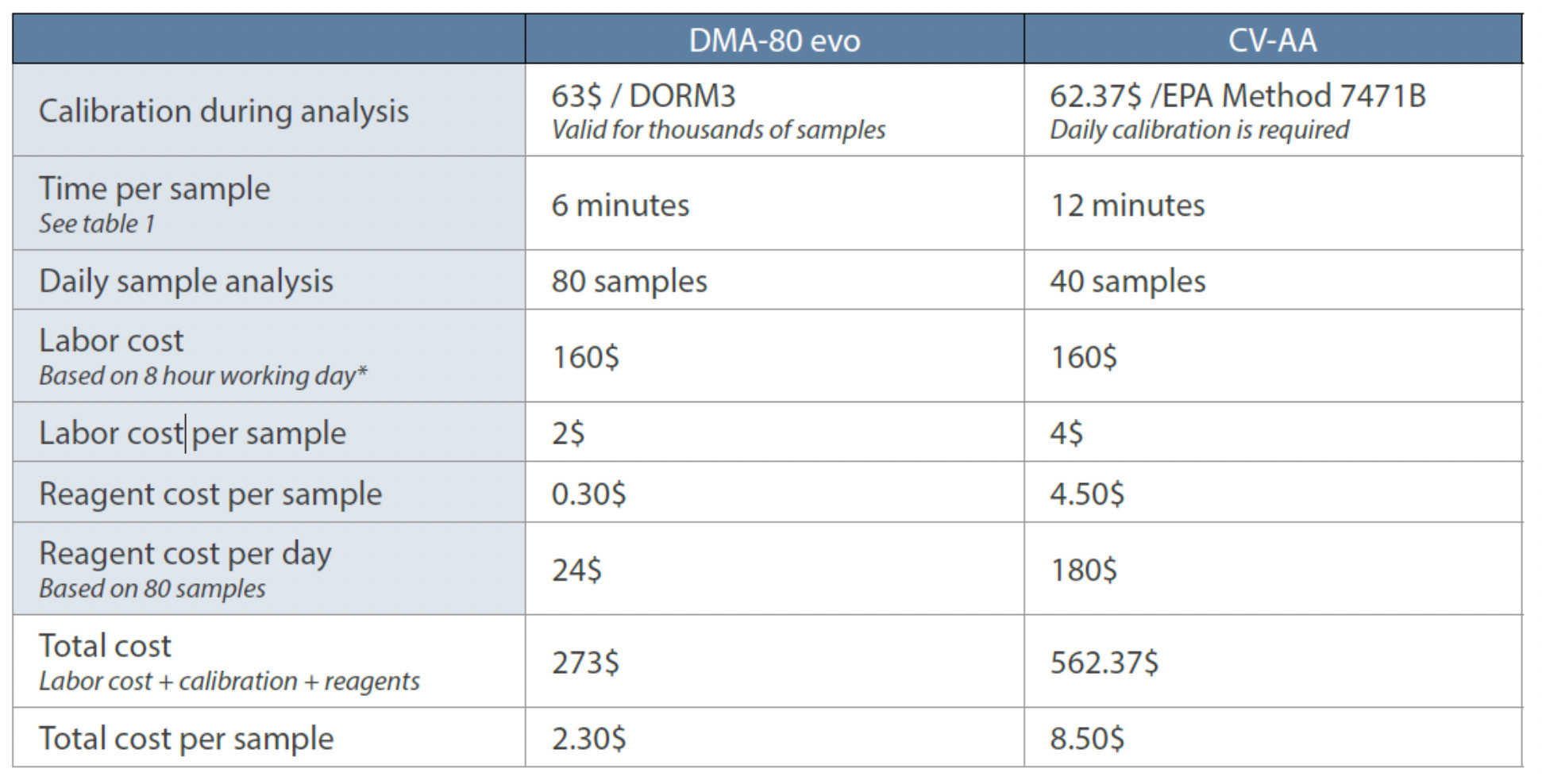
Table 1: Productivity comparison of DMA-80 evo with CVAA.
Productivity
The DMA-80 evo offers a significant enhancement in productivity over ICPMS and cold vapor systems. Not only do these techniques require labor-intensive and time-consuming sample preparation steps, which can lead to mercury losses, but also all the hazardous waste chemicals and solutions must be disposed of in a safe manner. In addition, ICP-MS and ICPOES are very prone to mercury carry over, as mentioned previously.
To avoid this problem the user typically has to dilute the sample, degrading the detection capability. In extreme cases with high levels of contamination, users have to clean the instrument using a gold solution or, in a worst case scenario, replace the sample introduction components.
All of these measures have a significant impact on sample throughput and productivity. The enhancement in productivity of the DMA-80 evo over CVAA is demonstrated in Table 1, which shows a factor of 4xdifference in the cost per sample. Thistranslates into total savings of almost $500 per day or $10,000 per month.
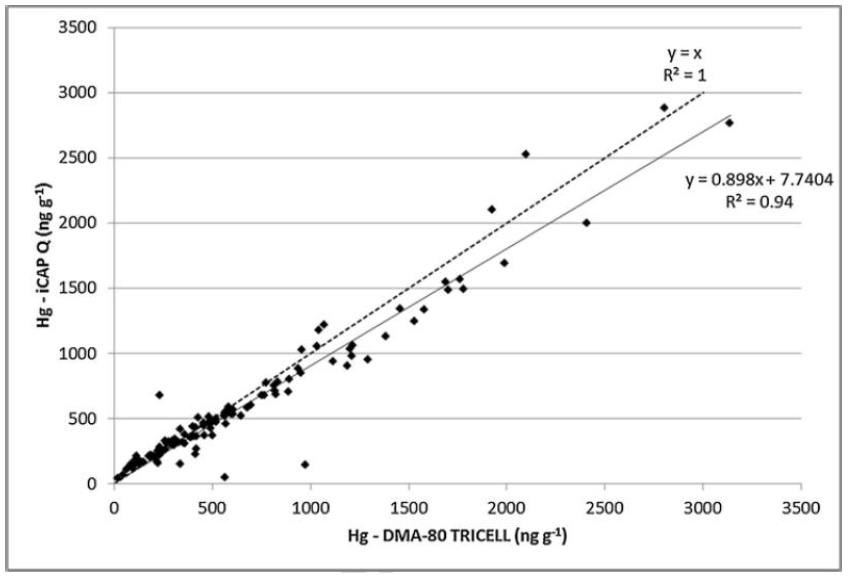
Figure 3: Correlation between quadrupole ICP-MS and the DMA-80 for the determination of Hg in hair (3)
Comparison with ICP-MS
A recent study by Domanico and co-workers at the University of Calabria in Italy compared thermal decomposition atomic absorption (DMA-80) with quadrupole-based ICP-MS (iCAP Q) for the quantitation of Hg in human hair (3). After the washing procedure to minimize the external contamination, two aliquots were taken from each hair sample.
The first was used for direct analysis of Hg using the DMA-80 and the second was digested for Hg determination by ICP-MS. Initial results indicated that the two data sets were fully comparable and were not statistically different. The two techniques demonstrated results with a good coefficient of determination (R2= 0.94) despite differences in dynamic ranges and method detection limits. Both techniques satisfied internal performance requirements and method validation resulting in sensitive, precise and reliable results as demonstrated in Figure 3.
However, the authors further concluded that the DMA-80 offered an attractive alternative to ICP-MS for hair analysis because of its reduced sample handling, lower risk of contamination due to the absence of the digestion step, less timeconsuming operation, and higher sample throughput, which results in a more costeffective
analysis. Let’s now take a closer look at some routine applications, which have been traditionally addressed by cold vapor AA/AF or ICP-MS.
Environmental Analysis
As more emphasis is placed on the monitoring of mercury emissions from power plants, there is an increasing demand to characterize soil, sediments and waste waters for remediation purposes. Several methods including ICP-MS and cold vapor are available for mercury analysis of these types of samples. However, both these techniques require complex sample preparation procedures that are labor intensive, time-consuming and subsequently expensive.
As a result, direct mercury analysis, as described in EPA Method 7473, was specifically developed for the rapid determination of mercury according to the Resource Conservation and Recovery Act (RCRA) governing the management of hazardous waste. Therefore, to exemplify its real-world capabilities the DMA-80 evo was used to carry out mercury determinations on a suite of soils, sludges and waste waters. In addition, two certified reference materials (NIST 2709 San Joaquin Soil CRM and BCR 277 Estuarine River Sediment CRM) were both analyzed at multiple times during the analytical run for quality assurance/ control purposes.
A subset of these data is exemplified in Table 2, which shows five separate analyses of the soil and estuarine sediment CRMs, together with the recovery and precision of a waste water sample spiked with 1 μg/L Hg. It can be clearly seen that the results and statistical data of both CRMs were in very good agreement with the certificate values, which were analyzed by cold vapor isotope dilution (CV-ID) ICPMS, recognized as being one of the most precise techniques for mercury quantitation. It should also be emphasized that with the wastewater sample, a calibration blank was analyzed before and after the five wastewater samples and 1 μg/L spike, showing the capability of the double-beam system to achieve very low background levels even after a suite of complex, real-world samples.
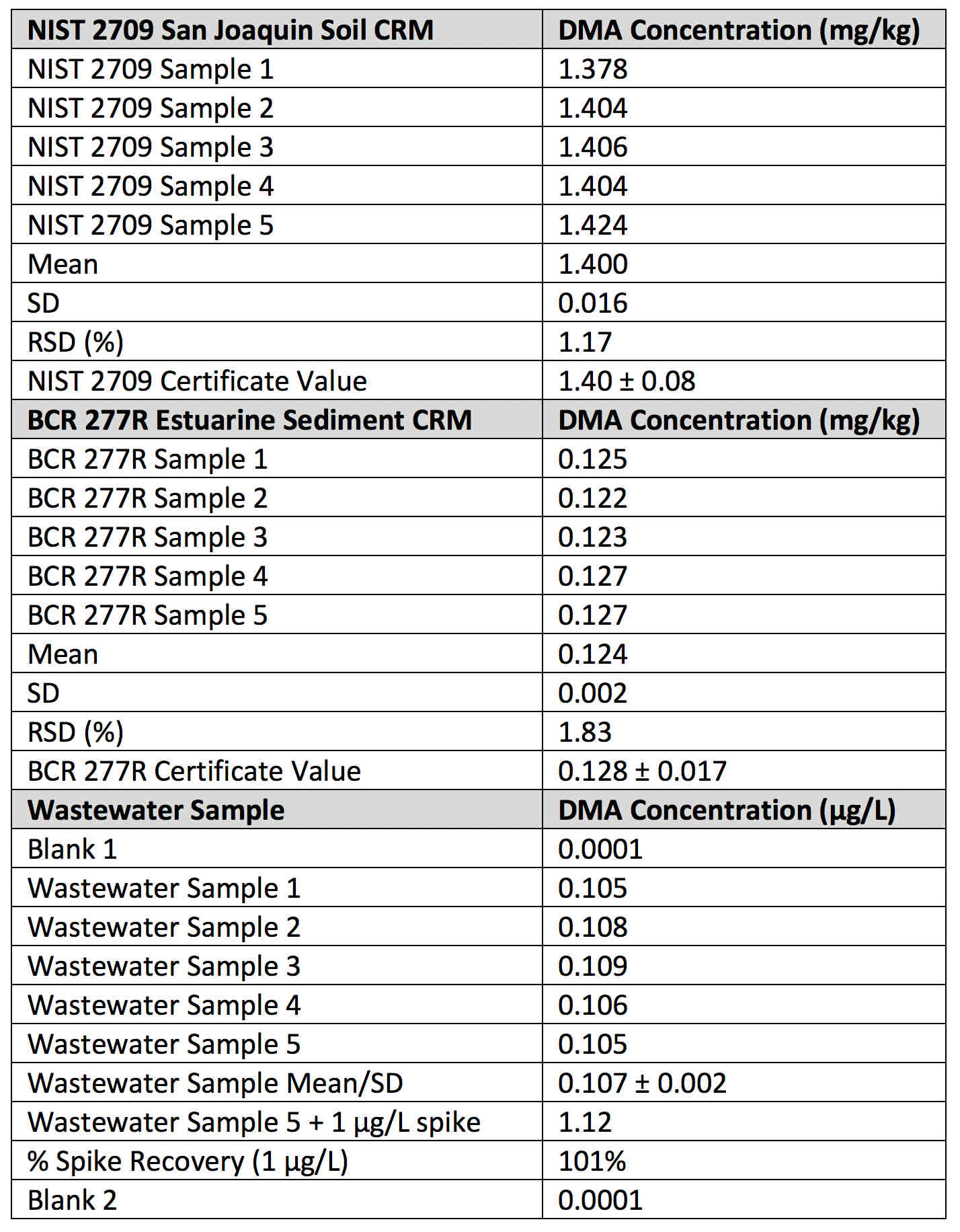
Table 2: Mercury results for NIST 2709 San Joanquin soil and BCR 277R Estuarine Sediment CRMs, together with the analysis of a wastewater samples spiked with 1 µg/L using the DMA-80 evo.
Petrochemical, Oil, Cement and Energy Industries
In the refining of crude oil, mercury is not only released into the environment during the refining process, but also from the combustion of refinery products such as gasoline and diesel fuel. Although mercury levels are typically less than 2 μg/kg in most crude oils, it has the potential to accumulate in equipment and cause operational problems in refining plants. It is therefore critical to monitor mercury to ensure operators are not exposed to the environmental impact of the mercury.
As a result, two analytical methods developed for the measurement of mercury in petrochemical samples are UOP 938-10 (Universal Oil Products) for total mercury and mercury species in liquid hydrocarbons (4) and the more recent ASTM D7623-10, a test method for total mercury in crude oil using combustiongold amalgamation and cold vapor atomic absorption (5).
However it is important to emphasize that if the quantitation of mercury species is not required, the preferred approach is the ASTM method. The cement industry is another segment that requires mercury monitoring. In their manufacturing process, limestone and other mineral components such as alumino-silicates and clays are fused together at high temperatures to form a clinker-type material, which is then ground up and mixed with other additives to make cement.
The heat required by this production process is typically achieved by burning coal or coke (a type of charcoal). However, due to high levels of mercury both in geological raw materials and fossil fuels, the production of cement clinker can potentially result in significant levels of mercury emission into the atmosphere. In fact, it is estimated that the total amount of mercury generated by the electricity and cement industries exceeds 60 tons per year combined (6).
For this reason, there is a growing demand for rapid and easy-to-use mercury analysis procedures. Direct mercury analysis is therefore a cost-effective alternative to traditional methods and has been used successfully to determine total mercury in a large variety of samples related to the generation of electricity and the production of cement.
A typical data set of these types of samples is shown in Table 3, which includes three different coke, coal, and limestone samples analyzed consecutively with blank levels in between. It can be seen quite clearly that the samples cover a wide range of mercury levels, from <1 μg/kg in the coke sample, to approximately 10 μg/ kg in the limestone, and up to about 30 μg/ kg in the coal sample.
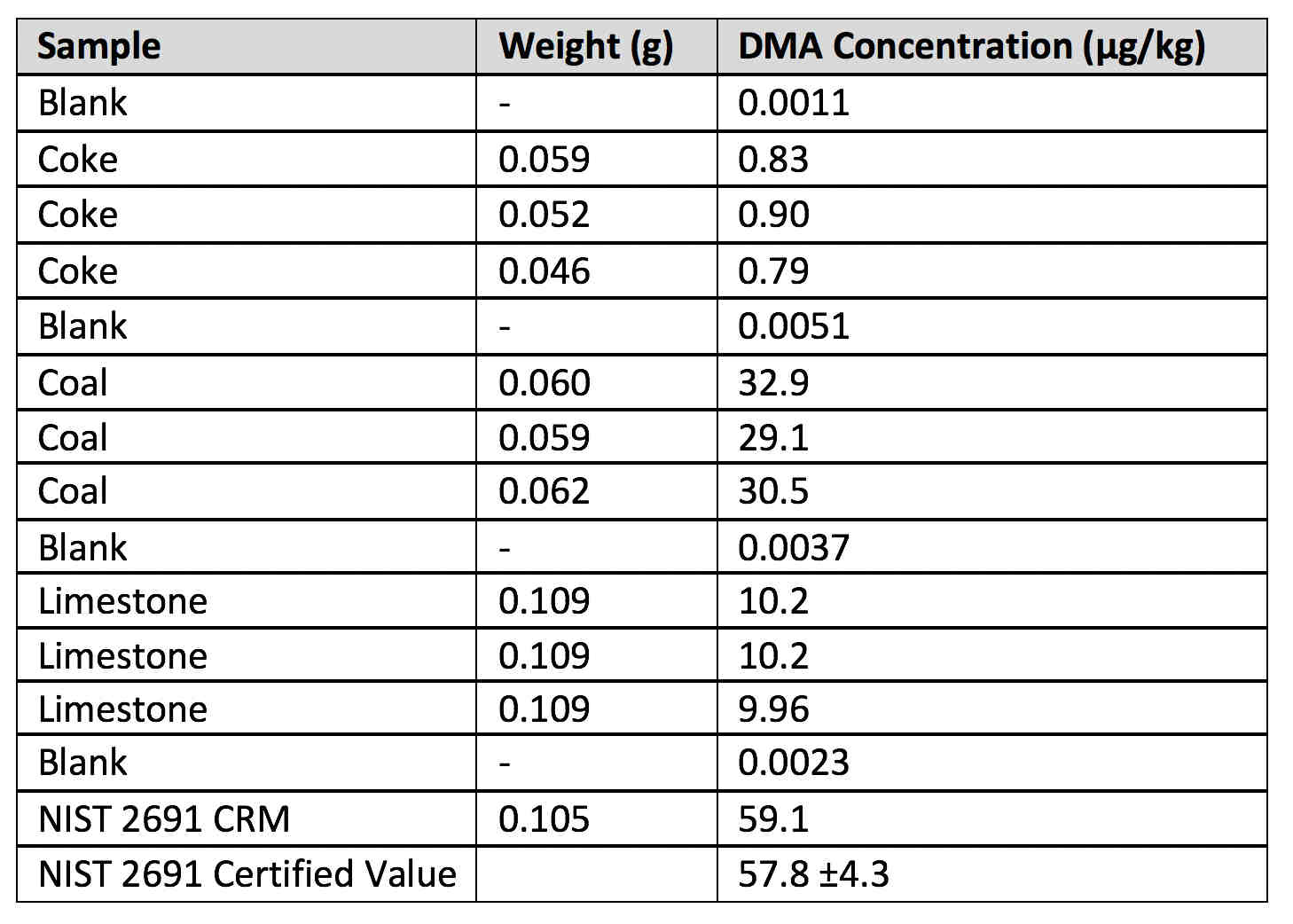
Table 3: Three different sample matrices (coke, coal, and limestone) analyzed consequetively with the DMA-80 evo, with blank levels in between and NIST 2691 coal fly ash CRM analyzed at the end of the run.
Two points should be emphasized. First, blank samples were analyzed at the beginning of the analysis, between each sample matrix and at the end of the analytical run. The second point is that NIST 2691 coal fly ash CRM was analyzed at the very end and achieved good accuracy compared to the certificate value, where the mercury was quantitated using CV-ID ICP-MS.
Conclusion
With over 2500 systems installed worldwide, direct mercury analysis has proved itself to be a viable complementary technique to CVAA and ICP-MS for carrying out mercury determinations. Its ability to analyze solids directly with minimal sample handling is very attractive for remote field based studies as well as for labs that don’tmhave sophisticated sample preparation and digestion procedures available.
In addition, the ability to introduce the sample directly into the instrument means contamination issues are minimized, which makes it ideally suited for non-experienced operators who are not versed in the finer points of analytical chemistry. And the DMA-80 evo with its new double-beam light path enhances its capability even further by compensating for any thermal and mechanical instability in the light source and optical components.Moreover, the Auto Blank feature goes a long way to ensuring that any abnormally high concentration samples do not negatively affect low samples that come immediately after it in the sample run.
With the recognized industry-leading application and service support of Milestone Inc. behind it, there is no question that the new DMA-80 evo is well-placed to take rapid, cost-effective and high productivity mercury determinations to the next level.
View Milestone DMA-80 evo listings on LabX.com
References
1. EPA Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, https:// www.epa.gov/homeland-security-research/epamethod-7473-sw-846-mercury-solids-and-solutionsthermal- decomposition
2. ASTM Method D6722-01: Standard Test Method for Total Mercury in Coal and Coal Combustion Residues by Direct Combustion Analysis, https://www.astm.org/DATABASE.CART/HISTORICAL/D6722-01.htm
3. F. Domanico et.al., J. Trace Elements in Med. and Biol. Vol 43, 3–8 (2017).
4. UOP 938-10: Total Mercury and Mercury Species in Liquid Hydrocarbons (2000); https://www.astm.org/ Standards/UOP938.htm
5. ASTM D7623-10: Standard Test Method for Total Mercury in Crude Oil Using Combustion-Gold Amalgamation and Cold Vapor Atomic Absorption (2025); https://www.astm.org/Standards/D7623.htm
6. Clean Air Act, 42 U.S.C. §7401 et seq., (1970); https://www.epa.gov/laws-regulations/summaryclean-air-act
Robert Thomas is the principal of Scientific Solutions, a consulting company that serves the application and writing needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 40 years and has written over 90 technical publications including a 15-part tutorial series on ICPMS. He recently completed his fourth textbook entitled Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide. He has an advanced degree in analytical chemistry from the University of Wales, UK, and is also a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem). He has led the heavy metals, plasma spectrochemistry task force on the ACS Committee on Analytical Reagents for the past 18 years.










