X-Ray Diffraction (XRD) for Crystalline Structure Analysis of Battery Electrode Materials
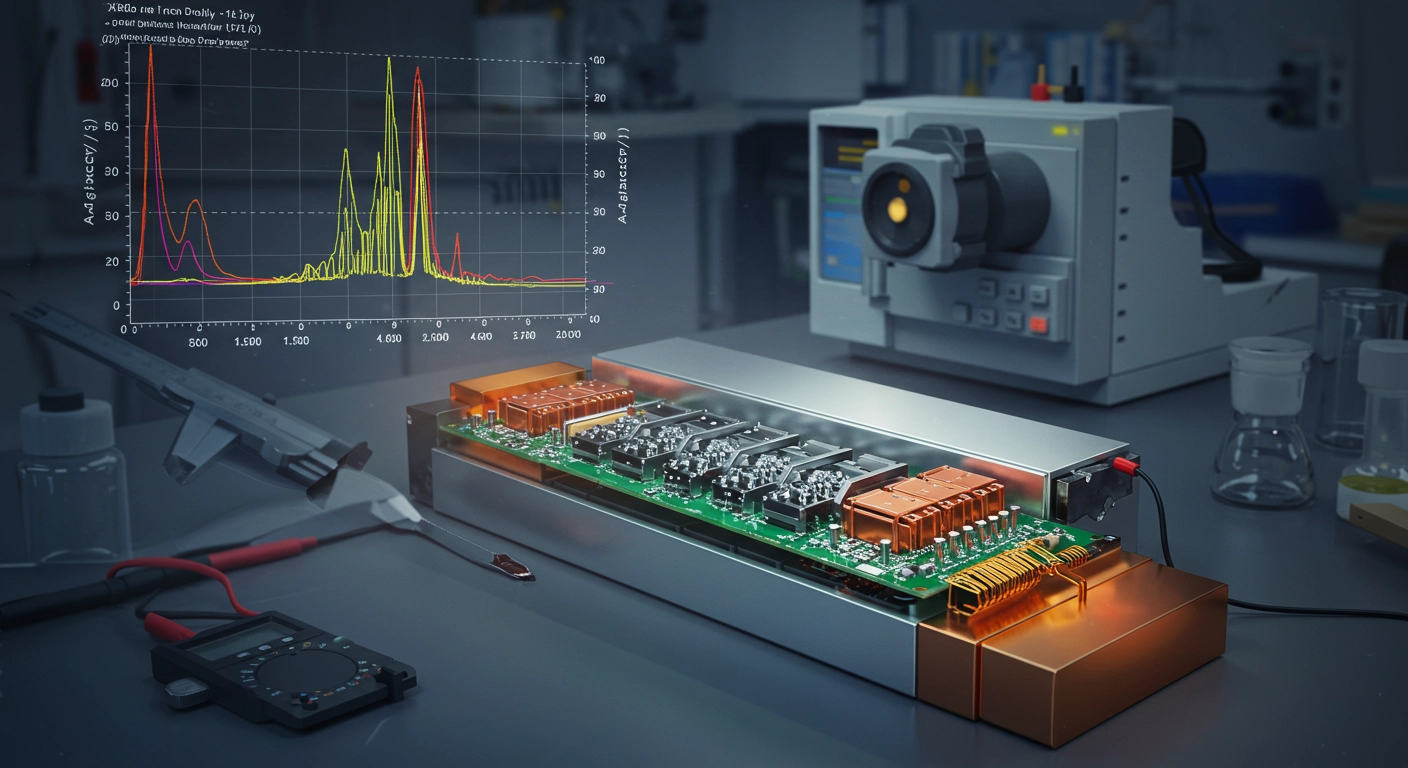
The relentless pursuit of more efficient, safer, and longer-lasting batteries is at the forefront of the global energy transition. From powering electric vehicles and portable electronics to enabling large-scale grid storage, the electrochemical performance of these crucial devices hinges significantly on the fundamental properties of their constituent materials, particularly the electrode materials. To truly optimize battery technology and push the boundaries of energy density, cycle life, and safety, scientists and engineers engaged in material science and characterization must possess a deep understanding of these materials at an atomic and molecular level. This is where X-Ray Diffraction (XRD) is an indispensable analytical technique, offering unparalleled insights into the crystalline structure of critical components like lithium-ion battery cathodes (e.g., LiCoO₂, LiFePO₄, NMC) and anodes (e.g., graphite, silicon-based compounds), thereby driving innovation in energy storage. A powerful, non-destructive analytical technique used for characterizing the crystallographic structure, chemical composition, and physical properties of materials. It operates on the principle that X-rays, when interacting with a crystalline material, are diffracted by the atoms in a regular, periodic manner. This diffraction pattern is unique to each crystalline substance, acting like a "fingerprint" that reveals its atomic arrangement. When a monochromatic X-ray beam strikes a crystalline sample, the X-rays are scattered by the electrons of the atoms within the crystal lattice. If the scattered waves are in phase, they constructively interfere, producing diffracted beams. Bragg's Law, By measuring the angles and intensities of these diffracted beams, researchers can deduce critical information about the material's internal structure. Battery electrode materials, such as lithium metal oxides (e.g., LiCoO₂, LiFePO₄, NMC) for cathodes and graphite or silicon-based compounds for anodes, are predominantly crystalline. Their electrochemical performance (capacity, voltage, cycling stability, rate capability) is intimately linked to their crystal structure. Any changes in this structure during synthesis, charging/discharging cycles, or degradation processes can profoundly impact battery performance. XRD provides vital information for: One of the primary uses of XRD is to identify the specific crystalline phases present in a battery electrode material. This is critical for: Confirming Synthesis: Verifying that the desired crystalline phase has been successfully synthesized and that no unwanted by-products are present. Detecting Impurities: Identifying any crystalline impurities that could negatively affect performance or safety. Monitoring Phase Transformations: Tracking phase changes that occur during electrochemical cycling (e.g., lithiation/delithiation processes in intercalation materials), which directly relate to charge/discharge mechanisms. XRD allows for the detailed determination of the crystal structure, including: Lattice Parameters: Precise measurement of the dimensions of the unit cell, which can change during cycling (e.g., volume expansion/contraction). Atom Positions: Locating the exact positions of atoms within the unit cell, providing insights into the bonding and structural stability. Site Occupancy: Determining how different atomic species occupy specific sites within the crystal lattice, crucial for understanding doping effects or cation mixing in layered cathode materials. The size of the individual crystallites (nanometer to micrometer scale) and the presence of microstrain within the material significantly influence its electrochemical properties: Crystallite Size: Smaller crystallites often lead to larger surface areas, which can improve ion diffusion kinetics and rate capability. XRD peak broadening is used to estimate crystallite size. Microstrain: Internal stresses or defects within the crystal lattice can impact ion transport and structural integrity. XRD peak broadening and shifting can reveal the presence and nature of microstrain. In some battery electrode manufacturing processes (e.g., thin film deposition, calendering), materials can develop a preferred crystallographic orientation (texture). XRD can quantify this texture, which can influence anisotropic properties like ion conductivity and mechanical stability. XRD is extensively used across various stages of battery research and development: New Material Discovery: Characterizing novel cathode, anode, and solid electrolyte materials to understand their fundamental structural properties and predict their electrochemical behavior. Process Optimization: Monitoring the effects of synthesis parameters (temperature, time, precursors) on the crystal structure and phase purity of electrode materials. In-situ/Operando XRD: Performing XRD measurements during battery operation (charging/discharging) to observe real-time structural changes, phase transitions, and lattice parameter variations, providing dynamic insights into reaction mechanisms and degradation pathways. This is particularly powerful for understanding the lithium intercalation/de-intercalation processes. Degradation Analysis: Investigating structural changes that lead to capacity fade, impedance rise, or safety issues in aged or failed batteries, helping to pinpoint degradation mechanisms. Quality Control: Ensuring batch-to-batch consistency in the crystal structure and phase composition of battery materials during manufacturing. X-Ray Diffraction is an indispensable tool in the arsenal of battery researchers. By providing detailed insights into the crystalline structure, phase composition, crystallite size, and strain of electrode materials, XRD enables scientists to: Design and synthesize new materials with enhanced performance. Optimize manufacturing processes for improved quality and efficiency. Unravel the complex mechanisms of battery operation and degradation. As the demand for advanced energy storage solutions continues to grow, the role of XRD will remain paramount, driving the fundamental understanding necessary to develop the next generation of batteries and power a sustainable future.What is X-Ray Diffraction (XRD)?
Why is XRD Crucial for Battery Electrode Materials?
1. Phase Identification and Purity
2. Crystal Structure Determination and Refinement
3. Crystallite Size and Strain Analysis
4. Texture and Preferred Orientation
Applications in Battery R&D
Conclusion: Powering the Future with Structural Insights










