A Cost-Effective, Ultra-Versatile Platform for High-Throughput Oligonucleotide Synthesis
The Shasta seeks to maximize reproducibility and yield while reducing resource consumption
The ability to run multiple protocols simultaneously can eliminate most of those variables, allowing researchers to have much greater confidence in their results. This concept was the inspiration for the development of new technology—The Shasta from Sierra Biosystems—that maximizes efficiency and reproducibility in high-throughput oligonucleotide synthesis.
The Challenges of Method Development
 Setting
up an oligo workflow involves many considerations. Will the system use closed
columns containing solid support? Or will it employ open columns in which
reagents are dispensed from overhead nozzles? Although closed-column systems
tend to give more reproducible results and higher yields, open-column systems
can generally achieve higher throughput.
Setting
up an oligo workflow involves many considerations. Will the system use closed
columns containing solid support? Or will it employ open columns in which
reagents are dispensed from overhead nozzles? Although closed-column systems
tend to give more reproducible results and higher yields, open-column systems
can generally achieve higher throughput.
Another consideration involves method optimization. To develop the most productive workflow, many conditions and variables must be tested. These include cross-experiment parameters to ensure each new set of data can be compared to data generated from another synthesis. Even slight deviations in the synthesis workflow can produce results that challenge the method optimization process.
This lack of efficiency in method development can lead to large deficiencies in the ability to get an oligo workflow up and running. Moreover, the need to balance reproducibility and yield with throughput is a challenge that most oligo synthesis labs must face, regardless of scale and budget.
The Shasta Solution
In contemplating these challenges, Bruce Erickson and colleagues at Sierra BioSystems engineered a novel 16-channel High-Throughput (HTP) oligonucleotide synthesizer—the Shasta. This system was developed with a dual emphasis on 1) enabling the user to conduct rapid protocol optimization as well as quick R&D experiments and 2) supporting HTP manufacturing of oligonucleotides. During validation, the Shasta synthesizer was used to test 60 protocols, optimizing 11 variables in just 4 runs. In addition to streamlined method development and increased throughput, the system demonstrated improved reproducibility and significantly reduced resource consumption.
Under the Hood of the Shasta
In its simplest configuration, the Shasta is a high-throughput oligo synthesizer capable of accepting 1 to 96 ABI-style synthesis columns or a 96-well plate. Alternative configurations allow for two 96-well plates (or 192 columns) or up to two 384-well plates. All initial validation experiments, as described below, used 96-well plates from Biocomma in the single-plate configuration.
The Shasta dispenses from a set of 64 nozzles (4 per reagent), using pressurized Argon to provide an anhydrous positive-pressure environment. A typical synthesis cycle step consists of:
- First, a Dispense step, in which the reagent is dispensed to the column or well.
- This is followed by a Pulse step, in which the reagent is briefly pushed into the solid support.
- Then, A Hold step that allows the reagent to slowly pass through the synthesis column, typically for 25-120 seconds, depending on the step.
- Finally, a Drain step pushes the remaining reagent through the solid support.
Columns or wells are divided into 16 independent Drain Groups, or “Banks,” which can be pulsed or drained independently. The use of multiple nozzles for each reagent allows each Bank to be treated independently of others, minimizing the potential for scheduling conflicts. When scheduling conflicts do occur, the speed at which reagents are dispensed ensures that no reagent can remain in contact with the solid support for more than 12 seconds longer than specified in the protocol.
Experimental Validation
To
validate the system, a representative 10-mer oligo (5’-dCATGTATGTC-3’) was used
as the primary test oligo to assess the performance and reproducibility of each
hardware configuration or synthesis cycle. This oligo was chosen because it is
a typical oligo, incorporating all four nucleotide bases, and is short enough
to allow HPLC and ESI LC-MS analysis. Testing with longer oligos, including
fluorescently labeled oligos, has since revealed that 10-mers are
representative of longer oligos regarding performance.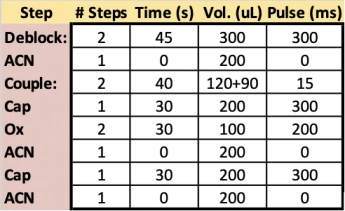
In method optimization experiments, three 96-well plates were synthesized at 200 nmol scale using modifications to the default standardized synthesis cycle (see Table 1; right).
Each synthesis used 16 unique protocols, varying three or four variables relating to a single step of the standard synthesis cycle (Deblock, Coupling, Capping, or Oxidation).
Fifteen test protocols were run per plate, using six replicates each, alongside a set of six positive control replicates.
Following an initial three runs, a fourth experimental plate was designed to test 15 additional protocols. These probed all four of the synthesis cycle steps to further reduce reagent consumption while maintaining high reproducibility.
Results
Deblock – Plate 1
 The
first run focused on the Deblock step and varied the reaction time, the
volume of Deblock, the number of Dispense steps, and the presence
or absence of a 100 ms Pulse step. The two best conditions, in terms of
both ASWY (average stepwise yields) and variability, used two block steps,
shorter reaction time (2x30s), and a single 100 ms pulse to push the reagent
into the CPG bed. Both the 200 µL dispense volume as well as the 150 µL volume
appeared ideal in generating oligos at 99.4% ASWY reproducibility (Table 2).
The
first run focused on the Deblock step and varied the reaction time, the
volume of Deblock, the number of Dispense steps, and the presence
or absence of a 100 ms Pulse step. The two best conditions, in terms of
both ASWY (average stepwise yields) and variability, used two block steps,
shorter reaction time (2x30s), and a single 100 ms pulse to push the reagent
into the CPG bed. Both the 200 µL dispense volume as well as the 150 µL volume
appeared ideal in generating oligos at 99.4% ASWY reproducibility (Table 2).
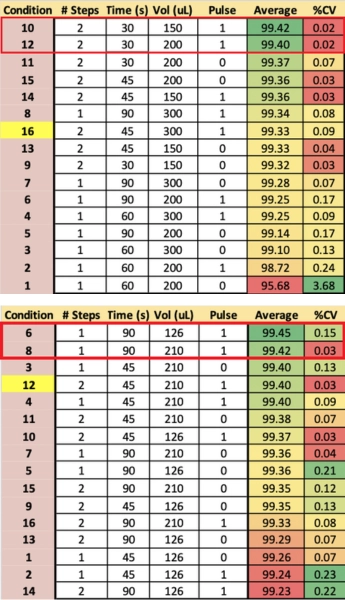
Coupling – Plate 2
The second experiment focused on the Coupling step. The reaction time was varied along with the volume of activator and phosphoramidite, the number of dispense steps, and the presence or absence of a 15 ms “pulse” step. In the run results, conditions 6 and 8 exhibited the highest yields (Table 3). Of this pair, only condition 8 had low variance. The 210 uL reaction volume was conducive to consistency without loss due to excessive draining and sample drying.
Cap/Ox – Plate 3
The third optimization experiment varied Cap/Ox order, the number of each step, the volume, and reaction time. Results showed nearly all reactions displayed both high ASWY and low %CV when used in conjunction with Oxidation steps sandwiched between two Cap steps. The highest yield and lowest variability resulted from Two Oxidation steps (between Cap steps), 120 uL volume, and 40 sec reaction time.
Cap/Ox Refinements – Plate 4
The fourth experiment focused on the best reaction conditions from the previous three plates, attempting to further reduce reagent consumption. Specifically, more restrictive conditions were varied for Deblock, Coupling, and both Oxidizer and Capping reagent time, frequency, sequence, and volume.
Conclusions
When performing synthesis cycle optimization, experiments are traditionally plagued by run-to-run variability issues despite proper control of all relevant variables. By removing the major source of variability and enabling the testing of up to 16 different protocols on the same plate in parallel, many of these issues can be eliminated. The result is the collection of actionable data that can be achieved even when running fewer replicates. As proof of concept, the experimental method optimization results described above were collected in one week, testing 60 different protocols in just 4 runs. This accelerated experimental workflow led to a significant reduction in reagent consumption while maintaining high coupling yields and reproducibility.
As any oligonucleotide chemist will attest, measuring significant differences between two protocols can be extraordinarily difficult. The Shasta has enabled high-yield method optimization beyond the results shown above, with the use of large numbers of replicates and greater statistical power. These further refinements of the Shasta platform—integrated into both the physical hardware and synthesis cycles demonstrate the true potential of this high-throughput, high-efficiency oligonucleotide synthesis solution.
Learn More about the Shasta at: https://sierrabio.com/
Read the application note: Rapid Oligonucleotide Synthesis Cycle Optimization to Reduce Reagent Consumption while Maintaining High Average Stepwise Yields and Maximizing Reproducibility
This editorial was published in partnership with Sierra Biosystems










