The Critical Role of Moisture Analysis in Lithium-Ion Battery Manufacturing and Electrolyte Quality Control

ImageFX (2025)
For laboratory professionals immersed in the intricate world of lithium-ion battery manufacturing and material science, the purity of components is paramount. Among the most insidious contaminants, moisture stands out as a silent saboteur, capable of wreaking havoc on battery performance, longevity, and, critically, safety. Even trace amounts of water can trigger a cascade of undesirable electrochemical reactions, leading to significant defects and premature battery failure.
The extreme sensitivity of lithium-ion cells to moisture necessitates rigorous moisture analysis and stringent control throughout every stage of the manufacturing process, from raw material procurement to final cell assembly. This article will delve into why moisture is such a critical concern, explore the essential analytical techniques employed to quantify it, discuss strategic moisture control measures, and highlight its profound impact on electrolyte quality and overall battery performance. Understanding and mastering moisture management is not merely a quality control measure; it is a fundamental pillar for producing reliable, high-performance, and safe lithium-ion batteries.
Why Moisture is a Critical Contaminant in Lithium-Ion Batteries
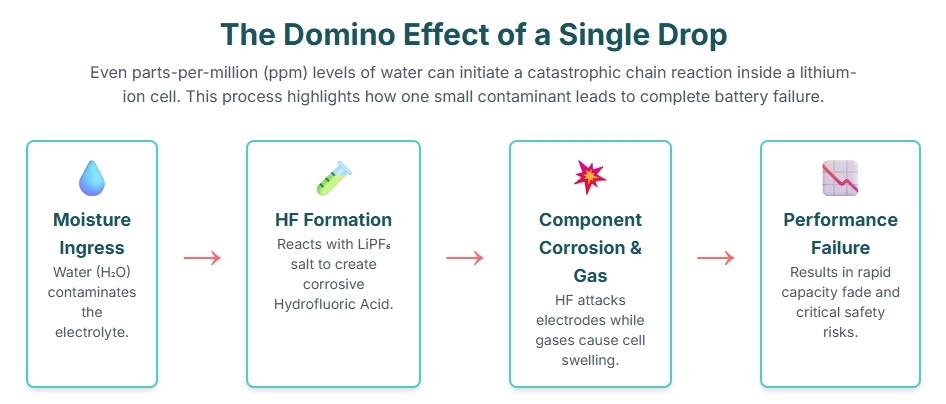
The detrimental effects of moisture in lithium-ion batteries stem from its highly reactive nature with key battery components, particularly the electrolyte and lithium metal. Even parts per million (ppm) levels can initiate a series of irreversible reactions that compromise cell integrity and performance.
Electrolyte Decomposition and HF Formation: The most significant and immediate reaction is with the lithium salt (e.g., LiPF₆) in the electrolyte. Moisture reacts with LiPF₆ to form hydrofluoric acid (HF).
LiPF6+H2O→LiF+2HF+POF3 HF is highly corrosive and attacks the active electrode materials, current collectors, and separator, leading to irreversible damage and accelerated degradation.
Gas Generation and Cell Swelling: Reactions with moisture can produce various gases, including CO₂, CO, and H₂. This gas generation leads to internal pressure buildup, causing cell swelling (pouch cell puffing) or even rupture in rigid cases, posing significant safety risks.
Solid Electrolyte Interphase (SEI) Layer Instability: The SEI layer, a crucial passivation layer formed on the anode surface during the first charge, is highly sensitive to moisture. Moisture can disrupt the stable formation of the SEI, leading to a porous, unstable, and resistive layer. This consumes active lithium, increases internal resistance, and accelerates capacity fade.
Lithium Metal Reaction: In cells utilizing lithium metal anodes (e.g., future solid-state or Li-metal batteries), moisture reacts violently with lithium, forming lithium hydroxide (LiOH) and hydrogen gas, further exacerbating safety concerns and consuming active material.
Increased Self-Discharge: Moisture-induced side reactions can create pathways for internal short circuits or parasitic reactions, leading to increased self-discharge rates and reduced shelf life.
Given these severe consequences, preventing moisture ingress and meticulously controlling its presence are non-negotiable requirements for battery manufacturers.
Key Moisture Analysis Techniques for Battery Materials
Precise quantification of moisture content is indispensable for quality control at every stage of battery production. Laboratory professionals rely on a suite of analytical techniques, each suited for different materials and moisture levels.
Karl Fischer Titration (KFT)
This is the gold standard for trace moisture analysis, particularly for liquid electrolytes and raw materials.
Principle: Based on the Bunsen reaction where iodine reacts with water in the presence of sulfur dioxide and a base. The amount of iodine consumed is directly proportional to the amount of water present.
Coulometric KFT: Generates iodine electrochemically. Ideal for very low moisture levels (ppm to sub-ppm) and small sample sizes.
Volumetric KFT: Adds a titrant containing iodine. Suitable for higher moisture contents (0.1% to 100%).
Application: Electrolytes, electrolyte components (solvents, salts, additives), electrode active materials (pre-drying), separators, and even residual moisture in assembled cells (via headspace analysis).
Advantages: High accuracy, high sensitivity, wide dynamic range, well-established method.
Limitations: Requires careful handling to prevent atmospheric moisture contamination, specific reagents, and trained personnel.
Dew Point Measurement
Crucial for monitoring the moisture content of inert atmospheres in dry rooms and glove boxes.
Principle: Measures the temperature at which water vapor in a gas condenses into liquid water (dew). A lower dew point indicates less moisture.
Application: Monitoring humidity levels in dry rooms (typically
−40∘C to−60∘C dew point or lower), glove boxes, and controlled atmosphere chambers used for electrode coating, cell assembly, and electrolyte filling.Advantages: Real-time, continuous monitoring, non-destructive.
Limitations: Measures moisture in gas, not directly in solid/liquid materials; sensor calibration is critical.
Loss on Drying (LOD) / Thermogravimetric Analysis (TGA)
Used for determining moisture content in solid materials.
Principle:
LOD: Measures the weight loss of a sample after heating it to a specific temperature until a constant weight is achieved. The weight difference is attributed to moisture and other volatiles.
TGA: Continuously measures the change in mass of a sample as a function of temperature or time in a controlled atmosphere. It provides a thermogram showing distinct weight loss steps for different volatile components, including moisture.
Application: Electrode active materials (cathode and anode powders), binders, separators, and other solid precursors.
Advantages: Simple (LOD), provides more detailed information on decomposition (TGA), suitable for larger samples.
Limitations: LOD cannot distinguish between moisture and other volatile organic compounds (VOCs); TGA requires careful interpretation to differentiate moisture from other thermal events.
Moisture Control Strategies in Manufacturing
Effective moisture analysis is only one part of the solution; stringent moisture control throughout the manufacturing process is equally vital. Battery production is typically carried out in highly controlled environments to minimize moisture ingress.
Ultra-Low Dew Point Dry Rooms: These specialized facilities maintain extremely low humidity levels (e.g., dew points below
−40∘C , sometimes as low as−70∘C ) to prevent moisture absorption by hygroscopic materials like electrode powders and separators. Personnel must wear specialized suits and follow strict protocols.Inert Atmosphere Glove Boxes: For highly moisture-sensitive steps, such as electrolyte filling and cell sealing, operations are performed in glove boxes filled with high-purity argon or nitrogen, maintaining sub-ppm levels of moisture and oxygen.
Material Handling and Storage: Raw materials, especially active electrode materials and electrolyte components, must be stored in hermetically sealed containers with desiccants and handled in controlled environments immediately upon opening.
Vacuum Drying: Electrode sheets and assembled cell components undergo extensive vacuum drying processes at elevated temperatures to remove residual moisture before electrolyte filling. This is a critical step to ensure minimal water content inside the final cell.
In-line Monitoring: Continuous, real-time moisture sensors are increasingly integrated into production lines to provide immediate feedback and identify potential moisture excursions.
These combined strategies form a robust defense against moisture contamination, safeguarding the quality and performance of lithium-ion batteries.
Impact of Moisture on Electrolyte Quality and Battery Performance
The electrolyte is arguably the most moisture-sensitive component in a lithium-ion battery. Its quality directly dictates the cell's electrochemical stability, ionic conductivity, and overall lifespan.
Electrolyte Degradation: As discussed, moisture leads to the formation of HF, which not only corrodes cell components but also degrades the electrolyte itself, reducing its ability to conduct ions efficiently. This results in higher internal resistance and reduced power capability.
Reduced Ionic Conductivity: Water molecules can interfere with the solvation of lithium ions, hindering their movement through the electrolyte. This directly impacts the battery's rate capability and overall performance, especially at high charge/discharge rates.
Compromised SEI Formation and Stability: A healthy SEI layer is crucial for long cycle life. Moisture in the electrolyte leads to a defective, porous, and unstable SEI. This unstable SEI allows continuous electrolyte decomposition and lithium consumption, accelerating capacity fade and increasing impedance.
Accelerated Capacity Fade: The cumulative effects of HF attack, gas generation, and SEI instability directly translate to a more rapid loss of usable capacity over cycling and calendar aging. Batteries with higher initial moisture content will invariably exhibit shorter lifespans.
Safety Implications: Electrolyte degradation due to moisture can lower the electrolyte's flash point or produce flammable gases, increasing the risk of fire or explosion, particularly during thermal runaway events.
Therefore, meticulous moisture analysis and control of the electrolyte are not just about performance; they are fundamental to the intrinsic safety and long-term reliability of lithium-ion batteries.
Conclusion: Moisture Analysis – The Unsung Hero of Lithium-Ion Battery Excellence
In the high-stakes arena of lithium-ion battery manufacturing, moisture analysis is not merely a procedural step; it is a fundamental guardian of quality, performance, and safety. The extreme sensitivity of battery components to even trace amounts of water necessitates a comprehensive approach, integrating advanced analytical techniques with stringent control measures throughout the entire production lifecycle.
By rigorously quantifying moisture content in raw materials, monitoring dry room environments, and implementing meticulous handling protocols, laboratory professionals play an indispensable role in mitigating the devastating effects of water contamination. Mastering these moisture management strategies is paramount for overcoming current challenges and unlocking the full potential of next-generation lithium-ion battery technologies, ensuring their continued evolution as a reliable and safe energy solution for a myriad of applications.
Frequently Asked Questions (FAQ)
Q1: Why is moisture considered a critical contaminant in lithium-ion batteries?
A1: Moisture is critical because it reacts with electrolyte components (like LiPF₆) to form corrosive hydrofluoric acid (HF), generates gases causing cell swelling, destabilizes the crucial SEI layer, and accelerates performance degradation and safety risks like thermal runaway.
Q2: What are the primary analytical techniques used for moisture analysis in battery materials?
A2: The primary techniques include Karl Fischer Titration (KFT) for highly accurate trace moisture in liquids, Dew Point Measurement for monitoring dry room and glove box atmospheres, and Loss on Drying (LOD) or Thermogravimetric Analysis (TGA) for moisture in solid electrode materials.
Q3: How does moisture specifically impact the electrolyte and battery performance?
A3: Moisture degrades the electrolyte by forming HF, which reduces ionic conductivity and attacks cell components. It also leads to an unstable SEI layer, causing increased internal resistance, accelerated capacity fade, and higher self-discharge rates, ultimately shortening the battery's lifespan.
Q4: What are key moisture control strategies implemented during battery manufacturing?
A4: Key strategies include conducting manufacturing in ultra-low dew point dry rooms and inert atmosphere glove boxes, strict material handling and storage protocols, extensive vacuum drying of components, and integrating in-line moisture monitoring systems to prevent contamination.










