Assessing Battery Safety: Using Thermal Analysis to Study Thermal Runaway and Exothermic Reactions
ImageFX (2025)
For laboratory professionals dedicated to the development and validation of lithium-ion batteries, safety is an overarching concern. While these energy storage powerhouses are integral to modern life, their inherent electrochemical nature carries the risk of thermal runaway – a self-accelerating exothermic reaction that can lead to catastrophic failure. Understanding and mitigating this risk is paramount, and this is where thermal analysis techniques become indispensable.
Thermal analysis provides the scientific tools to probe the thermal behavior of battery materials and cells under various conditions, from normal operation to severe abuse. By precisely identifying and quantifying exothermic reactions and their associated heat release, researchers can pinpoint critical safety thresholds, optimize cell designs, and develop robust battery management systems. This article delves into the principles of thermal runaway, explores the key thermal analysis techniques used to study it, and highlights how these insights are crucial for building safer, more reliable lithium-ion battery technologies.
Understanding Thermal Runaway: The Critical Safety Challenge
Thermal runaway is the most severe safety event in lithium-ion batteries, characterized by an uncontrollable rise in temperature that can lead to venting, fire, or even explosion. It's a chain reaction triggered by internal or external factors that cause the battery to generate more heat than it can dissipate.
The process typically unfolds as follows:
Initial Trigger: This could be an internal short circuit (due to manufacturing defect, dendrite growth), external short circuit, overcharge, rapid discharge, mechanical abuse (crushing, penetration), or exposure to high ambient temperatures.
Initial Exothermic Reactions: The trigger leads to initial exothermic reactions, such as the decomposition of the solid electrolyte interphase (SEI) layer on the anode, or reactions between the electrolyte and electrode materials. These reactions release heat, increasing the cell's internal temperature.
Temperature Escalation: As the temperature rises, it accelerates the rate of these initial reactions, leading to more heat generation. This positive feedback loop is the hallmark of thermal runaway.
Electrolyte Decomposition: At elevated temperatures (typically above
90∘C for common electrolytes), the electrolyte begins to decompose, releasing flammable gases and further contributing to heat generation.Separator Melting/Shrinkage: The polymer separator, which prevents direct contact between the anode and cathode, melts or shrinks (typically around
130−150∘C ), leading to internal short circuits and a rapid surge in current and heat.Cathode Decomposition: At even higher temperatures (e.g.,
180−250∘C for NMC, LCO), the cathode active material decomposes, releasing oxygen. This oxygen feeds the combustion of flammable electrolyte and anode materials, leading to a violent reaction.Cell Venting and Fire/Explosion: The rapid gas generation and heat buildup cause the cell casing to rupture, releasing hot, flammable gases and potentially leading to fire or explosion.
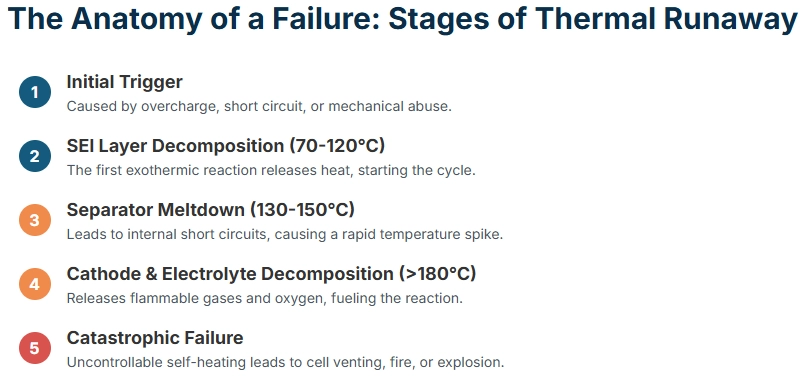
Understanding this sequence is fundamental to designing effective safety measures and is precisely what thermal analysis aims to characterize.
Key Thermal Analysis Techniques for Battery Safety Studies
Laboratory professionals employ specialized thermal analysis techniques to meticulously study the onset and progression of thermal runaway and other exothermic events. Each method offers unique insights into the thermal behavior of battery components and full cells.
Accelerating Rate Calorimetry (ARC)
ARC is the most widely used technique for simulating thermal runaway under near-adiabatic conditions, providing critical safety data.
Principle: The ARC operates in a "heat-wait-seek" mode. It heats the sample (battery cell) to a set temperature, waits for thermal equilibrium, and then "seeks" for any self-heating. If self-heating is detected, the calorimeter's heater tracks the sample's temperature, ensuring no heat escapes. This creates an adiabatic environment, allowing the direct measurement of the sample's intrinsic self-heating rate.
Key Parameters Measured:
Thermal Runaway Onset Temperature (
Tonset ): The lowest temperature at which the cell begins to self-heat uncontrollably.Maximum Self-Heating Rate (
dT/dtmax ): The fastest rate of temperature increase observed during the runaway event, indicating its severity.Total Heat Released (
Qtotal ): The cumulative energy released during the thermal event.
Application: Full cell safety testing under various abuse conditions (e.g., overcharge, external heating), evaluating the effectiveness of internal safety devices, and validating thermal models for Battery Management Systems (BMS).
Differential Scanning Calorimetry (DSC)
DSC is invaluable for identifying and quantifying exothermic and endothermic reactions of individual battery components.
Principle: DSC measures the difference in heat flow required to increase the temperature of a sample and a reference at the same rate. When the sample undergoes an exothermic (heat-releasing) or endothermic (heat-absorbing) reaction, this difference in heat flow is detected and recorded as a peak.
Key Parameters Measured:
Onset Temperature of Reaction: The temperature at which a specific reaction begins.
Peak Temperature: The temperature at which the reaction rate is maximal.
Enthalpy of Reaction (
ΔH ): The total heat absorbed or released during the reaction, quantified by the area under the peak.
Application:
Studying the thermal stability of electrode active materials (cathode, anode) with and without electrolyte.
Analyzing electrolyte decomposition reactions.
Evaluating the thermal behavior of separators (melting, shrinkage).
Screening new materials for potential thermal hazards.
Thermogravimetric Analysis (TGA)
TGA complements DSC by measuring mass changes as a function of temperature, providing insight into decomposition processes.
Principle: TGA continuously measures the mass of a sample as it is heated at a controlled rate in a specific atmosphere. Any mass loss indicates decomposition, evaporation, or desorption of volatile components.
Application:
Determining the decomposition temperatures of electrode materials, binders, and electrolyte components.
Quantifying the amount of volatile species released during heating.
Identifying the thermal stability limits of battery materials.
Synergy with DSC: Often used in conjunction with DSC (simultaneous TGA-DSC) to correlate mass loss events with heat flow events, providing a more comprehensive understanding of complex thermal reactions.
Characterizing Exothermic Reactions and Their Origins
The thermal stability of a lithium-ion battery is a complex interplay of various internal exothermic reactions. Thermal analysis techniques allow researchers to deconstruct these reactions and understand their contribution to thermal runaway.
Key exothermic reactions studied include:
SEI Layer Decomposition: The SEI layer, formed on the anode, can decompose at relatively low temperatures (e.g.,
70−120∘C ), releasing heat and potentially exposing fresh anode surface to electrolyte.Electrolyte Decomposition: The organic solvents and lithium salts in the electrolyte begin to decompose at higher temperatures (e.g.,
90−200∘C ), releasing flammable gases (e.g., CO, CO₂, H₂) and contributing significant heat.Anode Reaction with Electrolyte: The lithiated anode can react exothermically with the electrolyte, especially if the SEI layer is compromised.
Cathode Decomposition: The delithiated cathode material (e.g., LCO, NMC, NCA) is highly unstable at elevated temperatures (e.g.,
180−250∘C ), releasing oxygen that can fuel combustion of other components. This is often the most energetic reaction.Binder Decomposition: Polymeric binders used in electrodes can also decompose exothermically at certain temperatures.
By isolating and analyzing these reactions using DSC, and then observing their cumulative effect in a full cell using ARC, scientists can build a comprehensive thermal profile of the battery, identifying the weakest links in its thermal stability chain.
Integrating Thermal Analysis Data for Enhanced Battery Safety
The data gleaned from thermal analysis is not merely academic; it is directly actionable, driving critical decisions in battery design, material selection, and safety system development.
Material Selection: DSC and TGA data inform the choice of more thermally stable electrode materials, electrolytes, and separators, minimizing the potential for hazardous exothermic reactions.
Cell Design Optimization: Understanding heat generation and propagation allows engineers to optimize cell geometry, current collector design, and internal cell structure to improve heat dissipation and delay the onset of thermal runaway.
Battery Management System (BMS) Development: ARC data on
Tonset anddT/dtmax provides crucial parameters for developing sophisticated BMS algorithms. These algorithms monitor cell temperature and voltage, enabling the BMS to detect early signs of thermal instability and trigger protective measures (e.g., power reduction, cooling activation, disconnection) before thermal runaway occurs.Safety Device Integration: Insights into thermal behavior guide the design and placement of passive safety devices, such as current interrupt devices (CIDs) and pressure relief vents, ensuring they activate effectively to prevent catastrophic failure.
Regulatory Compliance and Certification: Thermal analysis data is often a mandatory requirement for battery safety certifications and regulatory approvals, demonstrating that battery products meet stringent safety standards.
Abuse Test Protocol Development: The understanding of thermal runaway mechanisms aids in designing realistic and effective abuse testing protocols that thoroughly challenge battery safety under extreme conditions.
By integrating these insights, thermal analysis serves as a cornerstone for developing the next generation of safer, more reliable, and higher-performing lithium-ion batteries.
Conclusion: Thermal Analysis – The Foundation of Next-Generation Battery Safety
In the dynamic landscape of lithium-ion battery technology, ensuring safety remains paramount. Thermal analysis techniques, particularly Accelerating Rate Calorimetry (ARC), Differential Scanning Calorimetry (DSC), and Thermogravimetric Analysis (TGA), provide the indispensable scientific foundation for understanding, predicting, and mitigating the risks associated with thermal runaway and exothermic reactions.
For laboratory professionals, mastering these analytical tools is not just about data collection; it's about gaining profound insights into the intrinsic thermal stability of battery materials and cells. By leveraging these insights, the industry can make informed design choices, develop more robust battery management systems, and ultimately accelerate the creation of safer, more reliable, and higher-performing lithium-ion batteries that power our world.
Frequently Asked Questions (FAQ)
Q1: What is thermal runaway in lithium-ion batteries?
A1: Thermal runaway is a self-accelerating exothermic reaction in a lithium-ion battery where internal temperature rises uncontrollably, leading to a cascade of reactions, gas generation, and potentially fire or explosion. It's triggered by abuse or internal defects.
Q2: How do Accelerating Rate Calorimetry (ARC) and Differential Scanning Calorimetry (DSC) differ in studying battery safety?
A2: ARC simulates worst-case thermal runaway in full cells under adiabatic conditions, measuring onset temperature and self-heating rate. DSC analyzes individual battery components, identifying specific exothermic reactions and their associated heat release, providing insights into material stability.
Q3: What are some key exothermic reactions that contribute to thermal runaway?
A3: Key exothermic reactions include SEI layer decomposition, electrolyte decomposition (releasing flammable gases), reactions between electrode materials and electrolyte, and cathode material decomposition (releasing oxygen), all contributing to heat generation and escalating the runaway process.
Q4: How does thermal analysis data help improve battery design and safety?
A4: Thermal analysis data informs the selection of more thermally stable materials, optimizes cell design for better heat dissipation, provides critical parameters for Battery Management System (BMS) algorithms to detect and prevent thermal events, and guides the integration of passive safety devices.










