Differential Scanning Calorimetry (DSC) for Phase Transitions and Thermal Behavior of Electrolytes and Separators
ImageFX (2025) For laboratory professionals at the forefront of battery development, the quest for higher energy density, faster charging, and extended cycle life is inextricably linked to ensuring paramount safety. As batteries operate, they generate heat, and their internal components—particularly the electrolyte and separator—must maintain stability across a wide range of temperatures. Understanding and controlling the thermal behavior of these materials is not just a performance metric; it's a critical safety imperative to prevent thermal runaway. This is where Differential Scanning Calorimetry (DSC) emerges as an indispensable analytical technique. DSC precisely measures the heat flow into or out of a sample as a function of temperature or time, revealing crucial information about phase transitions, thermal stability, and reaction kinetics. For electrolytes and separators, DSC provides vital insights into their melting points, glass transition temperatures, crystallization behavior, and decomposition pathways, directly informing material selection and battery design for next-generation energy storage solutions. Differential Scanning Calorimetry operates on a fundamental principle: it measures the difference in heat flow required to increase the temperature of a sample and a reference at the same rate. Both the sample and reference are kept at nearly the same temperature throughout the experiment. When the sample undergoes a thermal event (like melting, crystallization, or a chemical reaction), it either absorbs or releases heat, creating a difference in heat flow between the sample and the reference. This difference is precisely measured and plotted against temperature or time. Key thermal events revealed by DSC include: Endothermic Events: Processes that absorb heat (e.g., melting, evaporation, decomposition, glass transition). On a DSC curve, these appear as downward peaks. Exothermic Events: Processes that release heat (e.g., crystallization, curing, oxidation, decomposition). These appear as upward peaks. From these heat flow changes, DSC can quantify: Glass Transition Temperature ( Melting Point ( Crystallization Temperature ( Decomposition Temperature: The temperature at which a material begins to break down chemically. Enthalpy Changes ( By providing such detailed thermal fingerprints, DSC offers a powerful tool for material characterization and quality control in battery research. Electrolytes are the ion-conducting medium in batteries, and their thermal stability is paramount for both performance and safety. A stable electrolyte ensures efficient ion transport and prevents unwanted side reactions, especially at elevated temperatures. DSC is critical for: Phase Transitions of Solvent Components: Liquid electrolytes are typically mixtures of organic solvents. DSC can identify the melting and crystallization points of these individual components and their mixtures, which is crucial for determining the operating temperature range of the battery. For instance, a high freezing point could limit low-temperature performance. Glass Transition of Polymer Electrolytes: For solid-state batteries utilizing polymer electrolytes, the glass transition temperature ( Thermal Decomposition and Safety: Perhaps the most critical application of DSC for electrolytes is assessing their thermal decomposition. Exothermic peaks indicate reactions that release heat, which can lead to thermal runaway. DSC helps identify the onset temperature of these reactions and quantify the heat released, providing vital data for battery safety design. This includes reactions between the electrolyte and electrode materials at elevated temperatures. Heat Capacity ( By providing a comprehensive thermal profile, DSC enables researchers to select and formulate electrolytes that are stable and safe across the desired operating temperature range. The separator is a porous membrane that physically separates the anode and cathode, preventing electronic short circuits while allowing ion transport. Its thermal integrity is directly linked to battery safety. If the separator melts or shrinks excessively at elevated temperatures, it can lead to internal short circuits and thermal runaway. DSC is indispensable for: Melting Temperature ( Thermal Shrinkage Behavior: Beyond melting, excessive thermal shrinkage of the separator can expose electrodes, leading to direct contact and short circuits. While DSC directly measures heat flow, the melting point determined by DSC correlates strongly with the temperature at which significant shrinkage begins. Researchers often combine DSC with thermal mechanical analysis (TMA) for a full picture, but DSC provides the initial critical thermal stability data. Decomposition Temperature: DSC can identify the temperature at which the separator material itself begins to decompose, which is a critical safety limit. Compatibility with Electrolytes: Although not a direct measurement, DSC can be used to study the thermal interactions between the separator and electrolyte. For example, by analyzing the thermal behavior of a separator soaked in electrolyte, researchers can identify any exothermic reactions that might occur between the two components at elevated temperatures. Insights from DSC are vital for designing separators that maintain their structural integrity and insulating properties even under abusive thermal conditions, significantly enhancing battery safety. Beyond basic characterization, DSC offers advanced capabilities crucial for pushing the boundaries of battery research: Isothermal DSC for Long-Term Stability: Instead of scanning temperature, isothermal DSC holds the sample at a constant temperature and measures heat flow over time. This is invaluable for studying slow, long-term degradation processes or subtle exothermic reactions that might occur over extended periods, mimicking real-world battery aging. High-Pressure DSC: Some DSC instruments can operate under elevated pressure. This is particularly useful for studying reactions involving gas evolution (e.g., electrolyte decomposition) under conditions more representative of a sealed battery cell, where gas pressure can build up. Coupled Techniques (e.g., TGA-DSC): Combining DSC with Thermogravimetric Analysis (TGA) allows simultaneous measurement of heat flow and mass change. This powerful combination helps differentiate between endothermic/exothermic events caused by phase transitions and those caused by mass loss (e.g., solvent evaporation or material decomposition), providing a more complete picture of thermal events. Quantifying Reaction Enthalpies for Thermal Runaway: DSC can quantify the enthalpy ( These advanced applications allow researchers to delve deeper into the complex thermal dynamics within battery components, providing a more robust foundation for safety and performance optimization. The data obtained from DSC experiments directly translates into actionable insights for optimizing battery design: Material Selection: DSC helps in selecting electrolytes with broad thermal stability windows and separators with high melting points and minimal thermal shrinkage, ensuring robust performance across varying temperatures. Thermal Management System Design: Quantitative heat capacity and reaction enthalpy data from DSC are essential inputs for designing efficient battery thermal management systems (BTMS) that can dissipate heat effectively and prevent runaway. Predicting Lifespan and Safety: Understanding the thermal limits and decomposition pathways of components allows for more accurate predictions of battery lifespan under different operating conditions and helps identify potential safety risks associated with extreme temperatures. Quality Control and Manufacturing: DSC can serve as a quality control tool for incoming raw materials and manufactured components, ensuring consistency in thermal properties and adherence to safety standards. By integrating DSC insights into the design process, battery developers can create safer, more reliable, and higher-performing energy storage devices for a multitude of applications. In the relentless pursuit of advanced battery technologies, understanding and controlling the thermal behavior of internal components is non-negotiable. Differential Scanning Calorimetry (DSC) stands as a pivotal technique, providing an unparalleled ability to characterize the phase transitions, thermal stability, and decomposition pathways of critical materials like electrolytes and separators. From identifying the crucial shutdown mechanisms of separators to quantifying the exothermic reactions within electrolytes, DSC insights are fundamental to enhancing both battery performance and, most importantly, safety. By offering precise, quantitative thermal data, DSC is truly the thermal compass guiding laboratory professionals toward the development of the next generation of robust, safe, and high-performing energy storage solutions. A1: Differential Scanning Calorimetry (DSC) is used in battery research to measure the heat flow associated with thermal events in materials like electrolytes and separators, revealing their phase transitions, thermal stability, and decomposition temperatures crucial for battery performance and safety. A2: DSC helps assess separator safety by identifying its melting temperature (which indicates the shutdown mechanism to prevent short circuits) and decomposition temperature, providing critical data to prevent thermal runaway in batteries. A3: For electrolytes, DSC provides information on solvent melting/crystallization points, glass transition temperatures ( A4: Yes, by providing quantitative data on the thermal stability, phase transitions, and decomposition behaviors of battery components, DSC insights are invaluable for predicting battery lifespan under various thermal conditions and informing the design of effective thermal management systems to optimize performance and safety.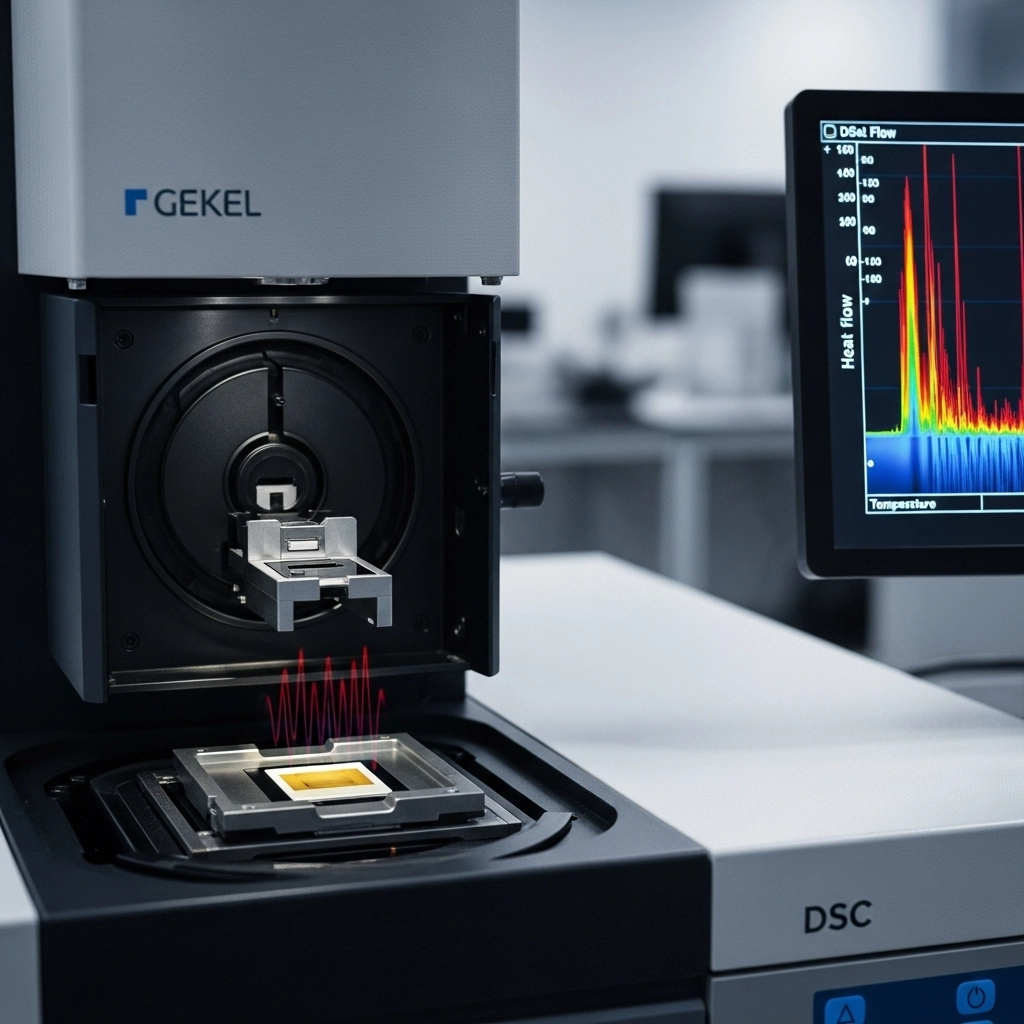
Understanding DSC: The Principle of Thermal Analysis
Characterizing Electrolyte Thermal Behavior with DSC
Assessing Separator Thermal Properties with DSC
Advanced DSC Applications in Battery Safety and Performance
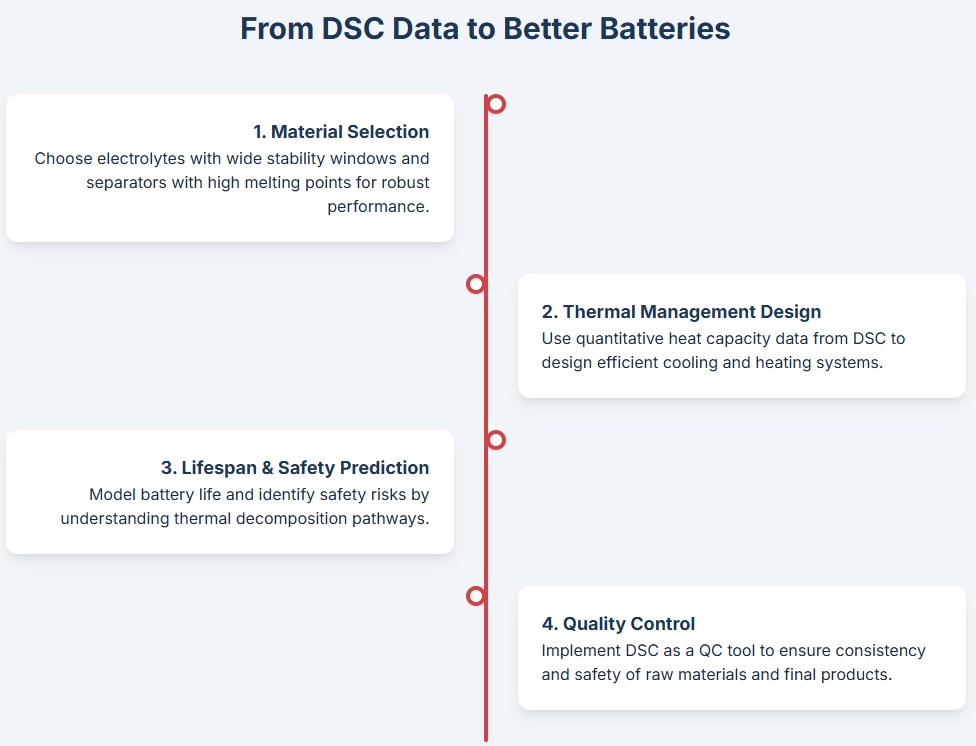
Optimizing Battery Design Through DSC Insights
Conclusion: DSC - The Thermal Compass for Next-Generation Batteries
FAQ
Q1: What is Differential Scanning Calorimetry (DSC) used for in battery research?
Q2: How does DSC help assess the safety of battery separators?
Q3: What kind of thermal information does DSC provide for electrolytes?
Q4: Can DSC be used to predict battery performance or lifespan?










