Insights on the use of ZipChip® CE-MS for Challenging Biotherapeutic and Proteomic Applications
Presentations at ASMS showcased the ZipChip technology in oligonucleotide, AAV, proteomics, and metabolomics investigations
 Protein
biotherapeutics, such as monoclonal antibodies, can be difficult to
characterize due their size and structural heterogeneity. Oligonucleotides
present challenges due to the presence of potential impurities and the need for
ion pairing reagents during analysis.
Characterization of gene delivery systems such as adeno associated virus
(AAV) is demanding due to the extent of protein complexity and limiting sample
volumes. Despite the challenges and structural diversity of these species, they
must be characterized to ensure their safety, efficacy, and potency during
production for therapeutic use.
Protein
biotherapeutics, such as monoclonal antibodies, can be difficult to
characterize due their size and structural heterogeneity. Oligonucleotides
present challenges due to the presence of potential impurities and the need for
ion pairing reagents during analysis.
Characterization of gene delivery systems such as adeno associated virus
(AAV) is demanding due to the extent of protein complexity and limiting sample
volumes. Despite the challenges and structural diversity of these species, they
must be characterized to ensure their safety, efficacy, and potency during
production for therapeutic use.
Capillary zone electrophoresis (CZE) is a proven separations technique that, when coupled with mass spectrometry (CE-MS), can enable identification of distinct charge variants and structural details of molecules. The implementation of microchip-enabled CE coupled with high-resolution mass spectrometry has proven to be an especially powerful approach, as demonstrated in a series of collaborative presentations showcased recently at the American Society of Mass Spectrometry (ASMS) conference.
What is ZipChip?
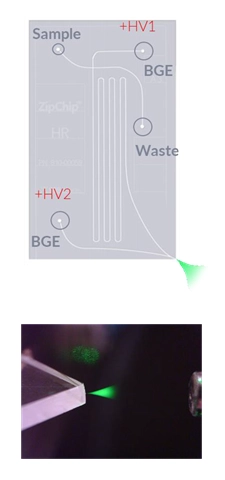 ZipChip®
from 908 Devices is an analytical separations device based on the concept of
microchip enabled microfluidics-based CE. The technology integrates sample
injection, electrophoretic separation, and electrospray ionization (ESI)
together in a microchip device that is coupled upstream of a mass spectrometer.
The refined separations system eliminates junctions, connections, and dead
volumes between individual functions, thereby supporting faster analysis times
and greater separation resolution.
ZipChip®
from 908 Devices is an analytical separations device based on the concept of
microchip enabled microfluidics-based CE. The technology integrates sample
injection, electrophoretic separation, and electrospray ionization (ESI)
together in a microchip device that is coupled upstream of a mass spectrometer.
The refined separations system eliminates junctions, connections, and dead
volumes between individual functions, thereby supporting faster analysis times
and greater separation resolution.
As shown in the diagram on the right, a sample is loaded and subjected to the electric field gradient. Following electrophoretic migration, the sample undergoes electrospray ionization and is guided into the mass spec (BGE: background electrolyte; +HV1: high-voltage 1, +HV2: high-voltage 2).
Several advantages of ZipChip make it ideal for challenging characterization workflows:
- The system houses a highly stable and uniform sample flow path for maximal separations efficiency.
- ZipChip separations require minimal sample prep, use low nanoliter sample sizes, and are compatible with multiple detergents and solvents.
- Since electrophoretic separations and nanoESI are integrated on the same microfluidic device, coupling with mass spec is straightforward.
- The platform is capable of simple workflows for diverse applications.
As showcased at the ASMS conference, ZipChip technology was recently implemented in several high-profile collaborations focused on monoclonal antibody, oligonucleotide, AAV, proteomic, and metabolomic investigations.
Glycosylation profiling of cetuximab subunits with CE-MS
Monoclonal antibodies can have numerous structural features and reactive groups making their characterization challenging. For example, lysine truncation or deamidation can create mass or charge variants, which may in turn impact the stability and bioactivity of the drug. Traditional techniques for assessing charge heterogeneity such as isoelectric focusing (IEF), imaged capillary isoelectric focusing (icIEF), and cation exchange chromatography (CEX) may be incompatible with MS for in-depth mass analysis. Capillary electrophoresis coupled with MS (CE-MS) offers the best performance for charge variant analysis and forms the foundation of the ZipChip separations technology.
As presented at ASMS, a collaboration between investigators at 908 Devices and Bruker Scientific explored glycosylation profiling of the therapeutic monoclonal antibody Cetuximab. This protein complex is known to possess four N-glycans with two located on each heavy chain in the FC and CH1 domains. Previous work has shown that the glycans are both heavily sialylated and complex, making their characterization difficult by typical methods such as reverse phase chromatography.
In the work, investigators performed limited proteolysis of Cetuximab antibody preparations and examined the products using a ZipChip coupled to a Bruker timsTOF MS tuned for intact and subunit mAb analysis. Bruker software was used for subsequent data processing and mass deconvolution.
Results showed the subunit charge variants were well separated with high-fidelity isotopic resolution, allowing confirmation of antibody subunits with sub-ppm mass accuracy. The electropherogram data demonstrated that basic subunit species migrated ahead of the acidic species. The acidic Fc subunits spectra were split into variants peaks, and high-resolution mass spectrometry (HRMS) data revealed glycan heterogeneity as the cause for these two populations. The basic Fd subunits were also split into two peaks. The delta mass of the peaks was indicative of sialylation corresponding to the difference between a galactose and a N-glycolyl neuraminic acid.
This work demonstrates the depth and resolution at which CE-MS can probe glycosylation profiles of Cetuximab and other biotherapeutics. Further evaluations are planned to explore potential differences between experimental data and published data, as well as to examine key differences between CE-MS and LC-MS analysis.
Capillary Electrophoresis Mass Spectrometry (CE-MS) of Oligonucleotides using a 908 Devices ZipChip
Ion-pair reverse-phase (IPRP) chromatography is the most common method for the characterization of oligonucleotides and the detection of impurities. The technique typically requires ion pair combinations such as triethylamine and hexafluoroisopropanol coupled with detection by negative ion mass spectrometry. Despite the success of this technique, ion-pairing reagents can contaminate the LC-MS system making it difficult to switch between applications. Often, a LC-MS system dedicated to a single workflow is used to avoid decontamination.
As highlighted at ASMS, a collaboration between investigators at 908 Devices and Boehringer Ingelheim Pharm sought to investigate the use of the ZipChip in concert with HRMS for oligonucleotide analysis in the absence of ion pairing reagents. Using a ZipChip coupled to a Thermo Lumos™ MS, siRNA samples were separated and analyzed in positive ion mode. Spectra was de-convoluted using the Oligo module software from Protein Metrics Inc.
Using these conditions, two unique peaks were resolved indicative of two strands from an original siRNA duplex. Although MS/MS and the use of collision induced dissociation (CID) is a standard method for obtaining sequence information from oligo fragmentation, modified oligos are typically more difficult to fragment using these standard methods. The investigators therefore used the higher-energy collisional dissociation (HCD MS) feature on the Thermo Lumos™ MS to successfully acquire high content fragmentation spectral data.
The work demonstrates the feasibility and improved performance of ZipChip CE-MS for ion painting reagent-free oligo characterization.
Rapid Characterization of Oligonucleotides and Related Impurities using Microchip CE-MS without Ion-pairing Reagents
With
growing interest in the use of oligonucleotides for therapeutics applications,
there is an increased need for improved techniques for production and quality
control. As mentioned previously, reverse phase LC utilizing ion pairing
reagents is the gold standard for oligo analysis. However, in addition to the
drawbacks of this method as mentioned above, ion pairing reagents can be
demanding on LC-
MS systems and can result in excess cleaning and potential downtime.
Presented at ASMS, a collaboration between 908 Devices and Thermo Fisher explored the use of a ZipChip in tandem with HR-MS on an Orbitrap Exploris™ 240 MS for oligo analysis in the absence of pairing reagents. The ZipChip Oligos Kit included a high-resolution bare glass (HRB) chip coupled with oligos background electrolyte (BGE).
Results demonstrated fewer charged states and simpler overall spectra compared to typical LC-MS methods. Full-length product (FLP) identifications were easily discernable from “shortmers”, or truncated standards spiked into samples at known concentrations. Forced degradation experiments showed the use of ZipChip can result in significant time savings for analyzing large sample cohorts. The method exhibited no carryover between oligo analysis runs, and the lack of ion pairing agents equates to minimal cleaning and associated downtime of the setup. Furthermore, the results demonstrate the same ZipChip setup can be used for multiple oligo analysis workflows.
Multi-level Expeditious Characterization of Adeno-Associated Virus (AAV) Capsid Proteins using ZipChip Microfluidic CE Coupled with MS
Recombinant adeno-associated virus (rAAV) constructs have emerged as promising delivery vectors for gene therapy and there is increasing interest in these recombinant vectors for use in vaccine development. Complete characterization of the viral capsid proteins is necessary to ensure safety and effective delivery in target systems. Challenges of this profiling include protein complexity of the capsid and limiting amounts of sample typically available for analysis.
In a project showcased at ASMS, investigators at 908 Devices in collaboration with Thermo Fisher explored the use of CE-MS for intact protein and peptide mapping of AAV capsid proteins. The work involved a ZipChip primed with background electrolyte , coupled to a Thermo Orbitrap Exploris™ 480 mass spectrometer. Peptide mapping entailed pepsin digestion followed by separation and analysis using full-scan MS and data-dependent MS/MS methods. Thermo Fisher Scientific BioPharma Finder™4.1 software was used for intact mass and peptide mapping data processing.
Peptide mapping experiments performed on 5-10 nL of the same starting sample yielded sharp electrophoretic peaks with peak widths ranging from 2-5 sec and a total CE-MS analysis time of less than 15 minutes. The method enabled separation and identification of low level post-translational modifications (PTMs) with better sequence coverage and 10-15-fold decreased analysis times compared with LC-MS/MS.
The approach is a fast and easy workflow that could be standardized for AAV capsid protein characterization.
Pushing the Boundaries of Speed and Sensitivity in Proteomics: Coupling SPE-Enabled Microfluidic Capillary Electrophoresis with Ultrafast Tandem Mass Spectrometry
The advantages of microchip CE-MS as a separation technique with high-efficiency and sensitivity are challenged by its limitation for use with low nL loading volumes. As a result, proteomic applications which require larger sample sizes and concentration ranges have not yet been compatible with CE-MS and instead have been relegated to more traditional LC-MS analytical approaches.
Scientists at 908 Devices sought to address this issue by engineering a 1 mm solid phase extraction (SPE) bed within the ZipChip to allow pre-concentration of samples prior to separations and analysis. The results include 100-fold improvements in concentration sensitivity limits. Loading capability is increased significantly, and peptide separations can be achieved on a 10-minute timescale at flow rates of ~100 nL/min and sample loadings of 0.1-100 ng.
As highlighted in a poster and platform talk at ASMS, 908 Devices, in collaboration with Thermo Fisher and the University of Washington, explored various MS acquisition speeds using a range of mass spectrometers to identify the optimal methods for high-performance, ultra-fast tandem MS-based proteomics. The pre-concentration method using the SPE functionalized ZipChip permits greater than 100 ng loading volume and greater than 110 peak capacities with a peptide separation window of ~ 7 min. The narrow window of peptide peak bases (2-3 seconds), however, presents a sampling issue for MS/MS when coupled to CE. Using dynamic, narrow width data-independent acquisition (DIA) methods, the investigators were able to combine the speed and resolution of microchip CE separations with fast sampling rates and nanoflow levels of sensitivity for MS/MS proteomics.
Future applications of this improved approach may include ultrafast analysis of sub-proteomes, online pre-enrichment for top-down proteomics, and single-cell proteomic analysis.
A Broad Application of Migration Time Indexing and Metabolite Libraries to Evaluate Quantitative Coverage in Microchip CE-MS Metabolomics
There is growing interest in the ability to perform metabolomic profiling on complex samples such as blood plasma. Such ability may lead to high-throughput analysis of important biological events in clinical settings. There are challenges with existing LC methods, however, which include complex, lower-throughput workflows and requirements for elevated sample input.
The microchip CE technique overcomes these issues by enabling increased separation efficiency and lower sample volume requirements. One current drawback of the approach involves higher migration time variability and drift in comparison with LC methods. Using the ZipChip, scientists at 908 Devices previously demonstrated improved accuracy for metabolite identification using indexed migration times (iMT).
Presented at ASMS and in collaboration with Thermo Fisher, 908 investigators applied iMT in the analysis of several metabolite libraries to evaluate the number of identifiable compounds in common metabolomics matrices.
Metabolite library standards (MSMLS: 700 compounds and AAPMLS: 156 compounds) were combined into 12 groups of non-isotopic molecules. A set of stable isotope labeled internal standards was added to each to enable quantification. Microchip CE-MS was performed using a ZipChip coupled with a Q Exactive HF or Exploris 240 Orbitrap MS instrument. Data analysis was performed using Skyline MLSDiscovery™ from IROA Technologies along with internal software.
The spectra exhibited unique iMT/accurate mass values which were used to quantify 177 compounds from the MSMLS Library and 128 compounds from the AAPMLS Library. The method required no additional metabolite derivatization and produced strong peak signals within a 4 min separation run.
Analysis of primary samples in the below matrices showed highly reproducible quantitation (252 compounds out of possible 322) with a separation time of 4 min under standard conditions. Analyses were performed using human and mouse plasma, human serum, various mouse tissues, and immortalized human cell lines. A variety of opioids of interest for drug screening were included in subsequent experiments with positive identification and quantification using standard methods.
This approach is efficient, allowing multiple analyses per sample without the need for separate sample extractions of polar, non-polar, or higher molecular weight analytes. In contrast, LC methods typically require specific workflows for different sample types. Furthermore, the high-throughput capabilities of the method may be compatible with automation, creating fast workflows for metabolite screening of clinical samples.
Summary
 Characterization
of therapeutic proteins, oligonucleotides, and viral vectors is essential for
validating their safety and efficacy for therapeutic applications. The use of
ZipChip CE-MS has many advantages for this characterization including the
ability to identify charge variants, discern structural details, and detect
potential impurities, among other attributes. Furthermore, proteomics and
metabolomics applications can be improved by expanding the number of
identifiable analytes in multiple sample types on fast time scales using low
sample volumes.
Characterization
of therapeutic proteins, oligonucleotides, and viral vectors is essential for
validating their safety and efficacy for therapeutic applications. The use of
ZipChip CE-MS has many advantages for this characterization including the
ability to identify charge variants, discern structural details, and detect
potential impurities, among other attributes. Furthermore, proteomics and
metabolomics applications can be improved by expanding the number of
identifiable analytes in multiple sample types on fast time scales using low
sample volumes.
The high-profile collaborations presented at ASMS showcase the ability of ZipChip technology to address challenging issues and push the field of microchip CE-MS into new territory and applications.
Download the PDF of this article
This editorial was written by LabX and published in partnership with 908 Devices










