Comparing Capillary Electrophoresis with HPLC: Efficiency and Resolution
GEMINI (2025)
The choice of separation technique is paramount for ensuring accurate, precise, and efficient analytical results in the modern laboratory. High-performance liquid chromatography (HPLC) and capillary electrophoresis are two foundational technologies that govern the analysis of myriad compounds, from small ions to complex biomolecules. Understanding the intrinsic differences between these two methodologies—particularly concerning their theoretical efficiency and practical resolution—is crucial for optimizing laboratory workflow and method development, ensuring the precise quantification and characterization of critical samples.
Fundamental Separation Mechanisms: High Resolution in Capillary Electrophoresis versus HPLC
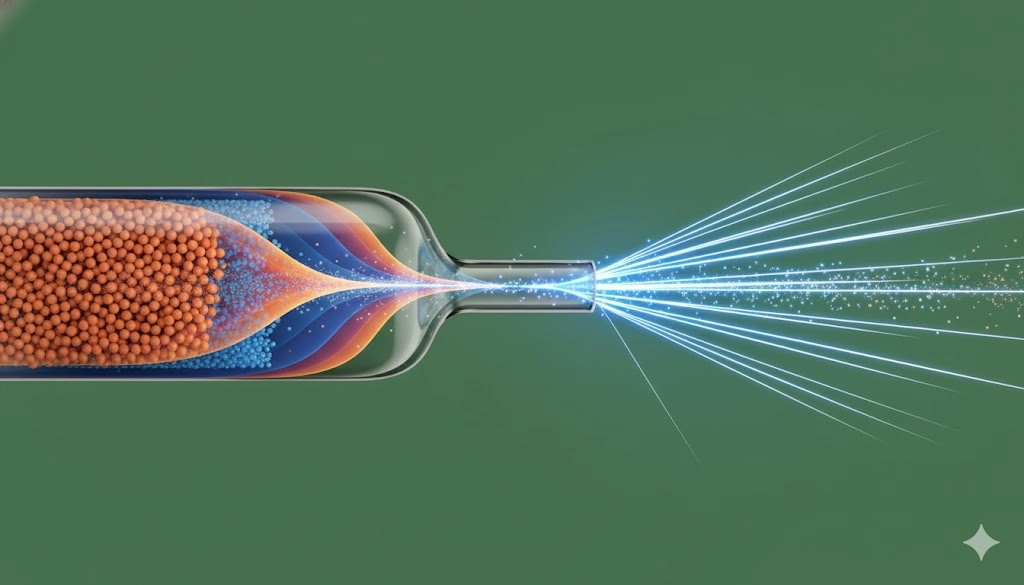
The core distinction between high-performance liquid chromatography (HPLC) and capillary electrophoresis lies in the driving force and the resulting flow profile within the separation medium. HPLC utilizes pressure-driven flow to push the mobile phase through a packed bed of stationary phase particles, while capillary electrophoresis (CE) relies on an electric field applied across a narrow-bore capillary tube containing an electrolyte buffer.
In HPLC, separation is governed by differential partitioning between the mobile phase and the stationary phase, leading to band broadening due to eddy diffusion, mass transfer resistance, and the non-uniform, parabolic flow profile inherent to pressure-driven liquid movement. The efficiency is therefore limited by the particle size and uniformity of the packed column.
In contrast, capillary electrophoresis employs electroosmotic flow (EOF) and electrophoretic migration to achieve separation. Because the electric field drives the entire bulk fluid movement (EOF), the flow profile within the capillary is virtually flat or "plug-like." This near-perfect flow profile dramatically minimizes the contribution of convection and parabolic flow to peak dispersion. The primary mechanism of band broadening in capillary electrophoresis is longitudinal diffusion, which, though present, is less significant than the multiple dispersion factors encountered in a packed HPLC column.
This difference in fluid dynamics provides capillary electrophoresis with an exceptionally high number of theoretical plates (N), often exceeding 100,000, and sometimes reaching millions, compared to the tens of thousands typically achieved by even the most efficient HPLC columns. This translates directly to superior resolution, especially for complex mixtures of similarly sized or charged analytes, such as resolving charge variants in therapeutic proteins.
Separation Technique | Driving Force | Flow Profile | Primary Separation Mechanism | Typical Theoretical Plates (N) |
|---|---|---|---|---|
HPLC | Pressure Pump | Parabolic (Laminar) | Partitioning (Chromatography) | 10,000 to 50,000 |
Capillary Electrophoresis | Electric Field | Plug-like (Uniform) | Electrophoresis and EOF | 100,000 to >1,000,000 |
Maximizing Analytical Efficiency and Minimizing Sample Consumption in Capillary Electrophoresis
Operational efficiency encompasses not just speed, but also the consumption of precious sample volume and reagents. The design of capillary electrophoresis systems inherently leads to advantages in these areas compared to traditional HPLC systems.
The small internal diameter of the CE capillary (typically 20–100 µm) means that the injection volumes are minute, often in the nanoliter or even picoliter range. This characteristic makes capillary electrophoresis the technique of choice for sample-limited applications, such as forensic analysis, single-cell analysis, or rare biomarker detection, where every microliter of sample is invaluable.
Furthermore, the high efficiency of capillary electrophoresis systems often translates to significantly reduced analysis times. While some ultra-high-performance liquid chromatography (UHPLC) methods can offer rapid separations, the inherent lack of column packing resistance in CE allows for the rapid application of very high voltages (up to 30 kV). This allows for rapid separations of complex matrices in under 15 minutes, whereas comparable resolution via HPLC might require lengthy run times or specialized, expensive columns.
Reduced solvent use is another critical efficiency consideration. HPLC requires large volumes of expensive, high-purity organic solvents for the mobile phase, which contribute significantly to operational cost and waste disposal. Capillary electrophoresis, on the other hand, uses aqueous-based electrolyte buffers. The consumption of these buffers is minimal—milliliters per day or less—dramatically lowering reagent costs, minimizing environmental impact, and simplifying laboratory waste management procedures.
Operational Costs and Instrumentation: Comparing HPLC and Capillary Electrophoresis Infrastructure
When considering the long-term viability and scalability of analytical methods, the instrumentation and associated running costs must be thoroughly evaluated. The infrastructure required for HPLC and capillary electrophoresis differs substantially in terms of initial investment, consumables, and maintenance complexity.
HPLC systems are robust and versatile, consisting of specialized modules: a high-pressure pump, autosampler, column oven, and a detector (UV-Vis, mass spectrometry, or refractive index). The primary consumable cost is the analytical column, which must be regularly replaced due to phase degradation or fouling, and the continuous consumption of high-purity solvents. Maintenance often involves pump seals, check valves, and intricate flow path components that operate under extreme pressure.
Capillary electrophoresis instrumentation is generally simpler and designed around the electrophoretic process. Key components include a high-voltage power supply, two buffer reservoirs, a cooling system (often air or liquid based), and an on-column or end-column detector. The main consumable is the fused-silica capillary, which is relatively inexpensive and easy to replace. While CE systems do require less intensive mechanical maintenance, method robustness can sometimes be a challenge due to the sensitivity of EOF to subtle changes in buffer composition, temperature, or capillary surface chemistry.
The capital expenditure for a foundational CE system can sometimes be lower than a fully modular HPLC or UHPLC setup. However, the total cost of ownership is generally lower for capillary electrophoresis due to:
Minimal organic solvent usage.
Lower consumption rate of inexpensive electrolyte buffers.
Lower cost of capillary consumables compared to high-end chromatography columns.
Reduced hazardous waste disposal costs.
Application Scope: Selecting Capillary Electrophoresis or HPLC for Biomolecules and Small Ions
The inherent strengths of high-performance liquid chromatography and capillary electrophoresis naturally dictate their most suitable applications. A laboratory's selection is often based on the analyte's properties (size, charge, hydrophobicity) and the required level of separation detail.
HPLC remains the standard workhorse for analyzing complex mixtures of non-polar or moderately polar small molecules, where hydrophobicity is the dominant separation characteristic. Common applications include:
Pharmaceutical active ingredient (API) and impurity profiling (e.g., reversed-phase HPLC).
Food safety testing (pesticides, toxins).
Environmental analysis (pollutants).
High-throughput quality control of bulk chemicals.
Capillary electrophoresis excels when the separation depends critically on the charge-to-size ratio of the analytes. It is particularly strong in areas where HPLC struggles to provide adequate resolution or efficiency, leading to the rapid adoption of capillary electrophoresis in the biopharmaceutical and chemical analysis sectors.
Key Strengths of Capillary Electrophoresis Applications:
Protein and Peptide Analysis: High-resolution separation of protein charge variants (CE-SDS, CIEF), peptide mapping, and analysis of post-translational modifications.
Chiral Separations: The use of charged cyclodextrin additives enables extremely efficient and cost-effective separation of enantiomers.
Inorganic Ion Analysis: Rapid analysis of anions and cations (Capillary Ion Analysis, CIA), often surpassing the speed and efficiency of traditional ion chromatography.
Nucleic Acid Analysis: High-resolution separation and sizing of DNA fragments (e.g., capillary gel electrophoresis).
While HPLC offers superior loadability and is often easier to interface with high-end mass spectrometry (MS) systems due to the high flow rate, capillary electrophoresis provides the requisite ultra-high resolution and is indispensable for charge-based separations and analysis of minute sample volumes.
Strategic Selection: Optimizing Laboratory Workflow with Capillary Electrophoresis and HPLC
The selection between capillary electrophoresis and high-performance liquid chromatography is a strategic decision that should be driven by the specific analytical challenge, sample availability, and operational efficiency goals of the laboratory. Capillary electrophoresis is clearly superior in terms of theoretical plate count, providing unparalleled resolution, and in terms of operational cost and sample conservation, due to its minute reagent and sample consumption. However, HPLC offers reliable method robustness, excellent scalability for preparative work, and compatibility with a wide array of existing detection technologies. The most efficient modern laboratories frequently employ both techniques, leveraging the high-resolution power of capillary electrophoresis for charge-based and sample-limited assays, while reserving HPLC for high-load, large-volume, and established small molecule chromatographic separations. Continuous investment in both methodologies ensures that laboratory professionals are equipped to tackle the full spectrum of analytical complexity.
Frequently Asked Questions about Capillary Electrophoresis and HPLC
What are the main limitations of capillary electrophoresis compared to HPLC?
Capillary electrophoresis often has limitations regarding sample loadability and detection sensitivity compared to high-performance liquid chromatography. The tiny internal volume of the capillary limits the amount of sample that can be injected, which can pose a challenge for trace analysis methods that require a large concentration factor.
Is high-performance liquid chromatography better than capillary electrophoresis for preparative separation?
Yes, high-performance liquid chromatography is generally superior for preparative-scale separations. The larger internal diameter of HPLC columns allows for the injection of significantly greater quantities of material, making it feasible to isolate compounds for downstream use, a task not typically possible with the minute volumes handled by capillary electrophoresis.
How does the cost of consumables compare between capillary electrophoresis and HPLC?
The consumables for capillary electrophoresis (capillaries and aqueous buffers) are typically much lower in cost than those for high-performance liquid chromatography (packed columns and organic solvents). This substantial difference contributes to a lower long-term running cost for capillary electrophoresis systems.
Which technique offers higher resolution for protein charge variants, capillary electrophoresis or HPLC?
Capillary electrophoresis, particularly techniques like capillary isoelectric focusing (CIEF), offers significantly higher resolution for separating protein charge variants than traditional HPLC methods. This high resolution is a direct result of the plug-like flow profile and the charge-based separation mechanism in capillary electrophoresis.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.










