Advanced NanoDrop Features Enable Sample Purity Analysis, Quality Control, and Regulatory Compliance
New NanoDrop instrument and software features are powerful solutions for today’s research, QA/QC, and regulated labs
The release of the NanoDrop over twenty years ago was a game changer for routine DNA, RNA, and protein analysis and quantitation. The revolutionary technology leverages sample droplet analysis for fast and easy concentration measurements using minimal amounts of sample.
The NanoDrop was developed to address pain points in molecular biology workflows such as time- and resource-consuming sample preparation and dilution. Several advanced features of the NanoDrop instruments, however, support high-level functions such as purity analysis, quality control, and compliance requirements. These capabilities extend well beyond routine nucleic acid concentration measurements, making the NanoDrop instruments and software indispensable for today’s research, quality control, and regulated labs.
Nucleic acid concentration measurement
 Nucleic acids have been traditionally quantified by determining
the UV absorbance at three analytical wavelengths: 230 nm, 260 nm, and 280 nm.
These absorbance measurements allow scientists to measure nucleic acid concentration
and have an indication of sample purity. The intensity of their maximum
absorbance peak at 260 nm is proportional to nucleic acid concentration.
Nucleic acids have been traditionally quantified by determining
the UV absorbance at three analytical wavelengths: 230 nm, 260 nm, and 280 nm.
These absorbance measurements allow scientists to measure nucleic acid concentration
and have an indication of sample purity. The intensity of their maximum
absorbance peak at 260 nm is proportional to nucleic acid concentration.
The advantages of this nucleic acid quantitation method are that it is simple, direct, and consumes only a small volume of your sample. One challenge, however, is its lack of specificity, as any contaminant that absorbs at these wavelengths will lead to inaccuracies in the resultant nucleic acid concentration. Other challenges with traditional techniques include the requirement for sample cuvettes and additional sample transfer steps. The detection range of traditional UV/Vis spectrophotometers often necessitates dilution and multiple measurements to arrive at accurate concentrations.
The NanoDrop solution
NanoDrop involves a unique sample retention system that employs surface tension to hold the sample droplet (1-2 µL) in place between two optical fibers. This format eliminates the need for sample cuvettes while allowing direct measurement of concentrated samples without the requirement for dilution. Sample consumption is minimized, and a wide range of detection ensures accurate and precise measurement of samples up to 200 times more concentrated than traditional cuvette-based UV/Vis methods.
The NanoDrop One/Onec has become a fixture in research labs due to its measurement accuracy and efficiency. The NanoDrop Eight excels by the ability to read up to 8 samples in parallel, thereby increasing precision and throughput. Both instruments produce data that can be maintained within 21 CFR Part 11 regulatory requirements.
How NanoDrop instruments can help you improve efficiencies
Improvements in lab efficiency start with time and resource savings. By avoiding the need for cuvettes, additional sample transfer steps, and sample dilutions, the Nanodrop instruments achieve significantly faster time-to-results. Reagent and sample use is minimized as well. Sample-to-sample precision is increased using minimal sample transfers and by avoiding multiple sample dilutions and replicates. The NanoDrop Eight instrument permits the measurement of up to eight samples simultaneously, which significantly increases precision and throughput for process-oriented applications.
NanoDrop onboard software enables fast, accurate data analysis of nucleic acids, labeled molecules, and other challenging molecules. As an example, the Microarray preconfigured method permits the measurement of up to two labeled nucleic acids in one step. Additional onboard methods are available for oligonucleotides, RNA, protein, cell cultures, and other analytes. Data can be printed or offloaded for storage or regulatory requirements.
How NanoDrop instruments can help you determine if your samples are pure enough for downstream experiments
 As mentioned above, a traditional drawback with absorbance
measurements is the lack of specificity. In the case of absorbance at 260 nm,
any molecule or contaminant that also absorbs at this wavelength will lead to inaccuracies
in the resultant nucleic acid concentration. Advances in UV/Vis software can
now selectively quantify nucleic acid absorbance in the presence of chemical
and nucleic acid contaminants.
As mentioned above, a traditional drawback with absorbance
measurements is the lack of specificity. In the case of absorbance at 260 nm,
any molecule or contaminant that also absorbs at this wavelength will lead to inaccuracies
in the resultant nucleic acid concentration. Advances in UV/Vis software can
now selectively quantify nucleic acid absorbance in the presence of chemical
and nucleic acid contaminants.
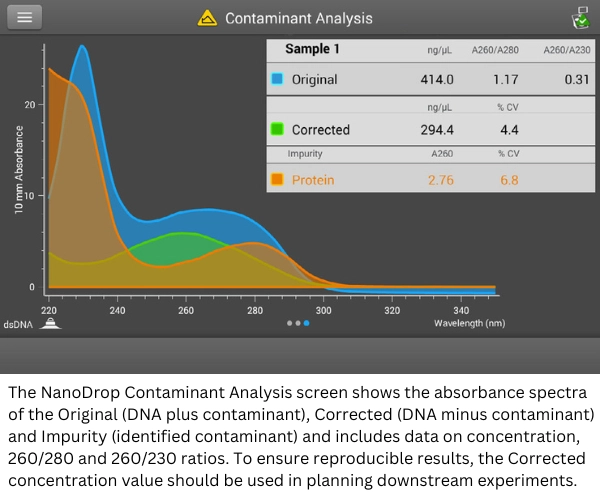
Thermo Scientific Acclaro Intelligence Technology software—which is standard in NanoDrop One/OneC and NanoDrop Eight spectrophotometers—uses full-spectrum data and advanced algorithms to identify common nucleic acid contaminants and provide corrected nucleic acid concentrations. The Acclaro software incorporates algorithms that rely on a reference library of contaminant-specific spectra. The software applies these algorithms to sample spectra and makes predictions about potential contaminants using chemometric mathematical principles.
The Acclaro reference libraries currently support the detection of protein, phenol, guanidine HCl, and RNA in dsDNA samples. The libraries also support the detection of protein, phenol, guanidine isothiocyanate, and dsDNA in RNA samples. The contaminant libraries are expanding with periodic updates.
Sample purity analysis provided by the Acclaro software helps to identify the specific contaminants present in the sample in order to minimize risk to downstream protocols. The analysis also helps provide an accurate, corrected concentration of analyte, which is critical when concentration is a major factor, such as in PCR or qPCR.
How NanoDrop instruments can increase sample quality control
In applications such as RT-qPCR, nucleic acid concentrations are critical, particularly at the enzymatic steps of the reaction. During reverse transcription, RNA concentrations must be controlled to minimize variability in cDNA production. During extension, DNA concentration must be sufficient to produce definitive results and minimize side reactions.
The MIQE guidelines—minimal information for publication of quantitative real-time PCR experiments—require that input RNA quantity and purity be reported for publications involving qPCR data. The Acclaro Sample Intelligence Technology built into the NanoDrop One and Eight instruments can provide corrected concentrations and identify the presence of contaminants that may impact results and data publication.
Protein quantification is enabled on the NanoDrop One/Onec using preprogrammed applications. Direct measurements can be performed by capturing absorbance at 280 nm and 205 nm and using the protein-specific coefficient. Colorimetric assays such as BCA, Bradford, Lowry, and Pierce 660 nm can also be performed as indirect measurement applications by forming a standard curve and calculating protein concentrations. The range of preconfigured applications allows an appropriate selection of protein quantification methods or multiple methods. This increases measurement efficiency while supporting increased sample quality control.
How NanoDrop software tools can support QA/QC labs and help maintain compliance with 21 CFR Part 11
Quality assurance and quality control (QA/QC) labs have an increased burden to ensure data is precise, reproducible, and reliable. The NanoDrop instruments are positioned to address these considerations by integrating next-generation sample quality assessment into QC/QA lab workflows.
In the case of emerging technologies such as mRNA vaccine quality control, QC analysis is critical at several stages of mRNA vaccine production. The sequencing stage requires high-quality material and accurate nucleic acid concentrations. Plasmid production must be monitored for truncations and other genomic contaminants. In vitro transcription can produce side reactions, and final mRNA preparations require purity analysis prior to vaccine testing.
Regulated environments such as pharmaceutical and biotechnology labs demand measures that ensure products are of the highest quality and are traceable in accordance with 12 CFR Part 11. The optional Thermo Scientific Security Suite or Thermo Scientific SciVault software helps to keep these labs compliant. Security Suite software on the NanoDrop One/Onec features includes user account access, audit trail capability, electronic signatures, automated data back-up, and other tools. SciVault software on the NanoDrop Eight instrument is equally capable.
Summary
The advent of NanoDrop technology changed the landscape of nucleic acid measurement and quantification. Beyond routine applications, advanced software capabilities now allow these instruments to perform purity analysis, enable quality control checks, and support regulated laboratory environments. What once was a game-changing tool in the lab is now an indispensable solution for a wide range of lab processes and applications.
This editorial was written by LabX and published in partnership with Thermo Scientific
To learn more about the Thermo Scientific NanoDrop family of instruments, please visit: www.thermofisher.com/NanoDrop










