The Future of Clonal Isolation: Automated Single-Cell Dispensers
Combining Microfluidics, Flow Cytometry, and
Liquid Dispensing to Address Challenges in Cell Line Development
In the relentless pursuit of life-saving breakthroughs, the adage "slow and steady wins the race" finds no place within the dynamic realm of drug discovery and development. The race against time and disease demands a paradigm shift—where efficiency and unwavering data reliability are the driving forces across every stage, from pioneering research to industrial-scale production.
While technological advances have graced every aspect of drug discovery and development today, challenges still abound, demanding newer tools and techniques that can further accelerate the process. This article briefly reviews current challenges in cell line development, specifically around clonal isolation, and how you can address these challenges.
What is Clonal Isolation?
Cell lines serve many purposes, from aiding academic biological research to being the building block for developing commercial therapeutics. A cell line needs to be genetically stable and homogenous to maintain permanent and stable expression of the desired protein/biomolecule. However, achieving such homogeneity requires several steps after the cells have been engineered: clonal isolation, clone screening, and a host of assays for confirmation of phenotype.
Because clones refer to groups of cells that are all genetically identical, they need to be derived from a single cell. Therefore, the group of cells that have been engineered need to be sorted through and analyzed individually. This process is called clonal isolation and is a fundamental step towards ensuring genetic uniformity. Clonal isolation also validates the relationship between the introduced genetic alteration and the observed phenotype and provides a reliable foundation for subsequent steps.
Current Techniques and Challenges of Clonal Isolation
Fluorescence-activated cell sorting (FACS) and limiting dilution are two techniques commonly used to achieve clonal isolation. FACS works by sorting cells based on their fluorescence properties. Once the cells are labeled with fluorescent markers, they are introduced into the FACS instrument and their fluorescence intensity is measured against specific criteria, which in turn determine whether cells should be isolated or not. The chosen cells are separated and dispensed into a collection tube or plate.
Notably, this traditional FACS process is carried out under high pressure (30-70 psi) and rapid flow, which potentially causes damage and stress response in cells, leading to low post-sort viability1. Furthermore, it is a highly expensive instrument and requires specialized expertise to run it. Clogging is a long-standing issue with traditional FACS due to the small dimensions of the flow cell and fluidic path. Unclogging can take away a significant amount of machine time and delay planned experiments. There is also an increased risk of contamination due to shared fluidics and operation in non-sterile environments.
Limiting dilution, on the other hand, is a gentler technique that works on the principle of decreasing cell concentration through serial dilution of suspensions. While cells aren’t subjected to high pressures or are dependent on their fluorescent properties, limiting dilution is often a manual and labor-intensive process that is highly prone to error. The probability of single-cell deposition using limiting dilution follows poison distribution, resulting in poor and unreliable efficiency in overall single-cell deposition2.
The Advantages of Automated Single-Cell Dispensers
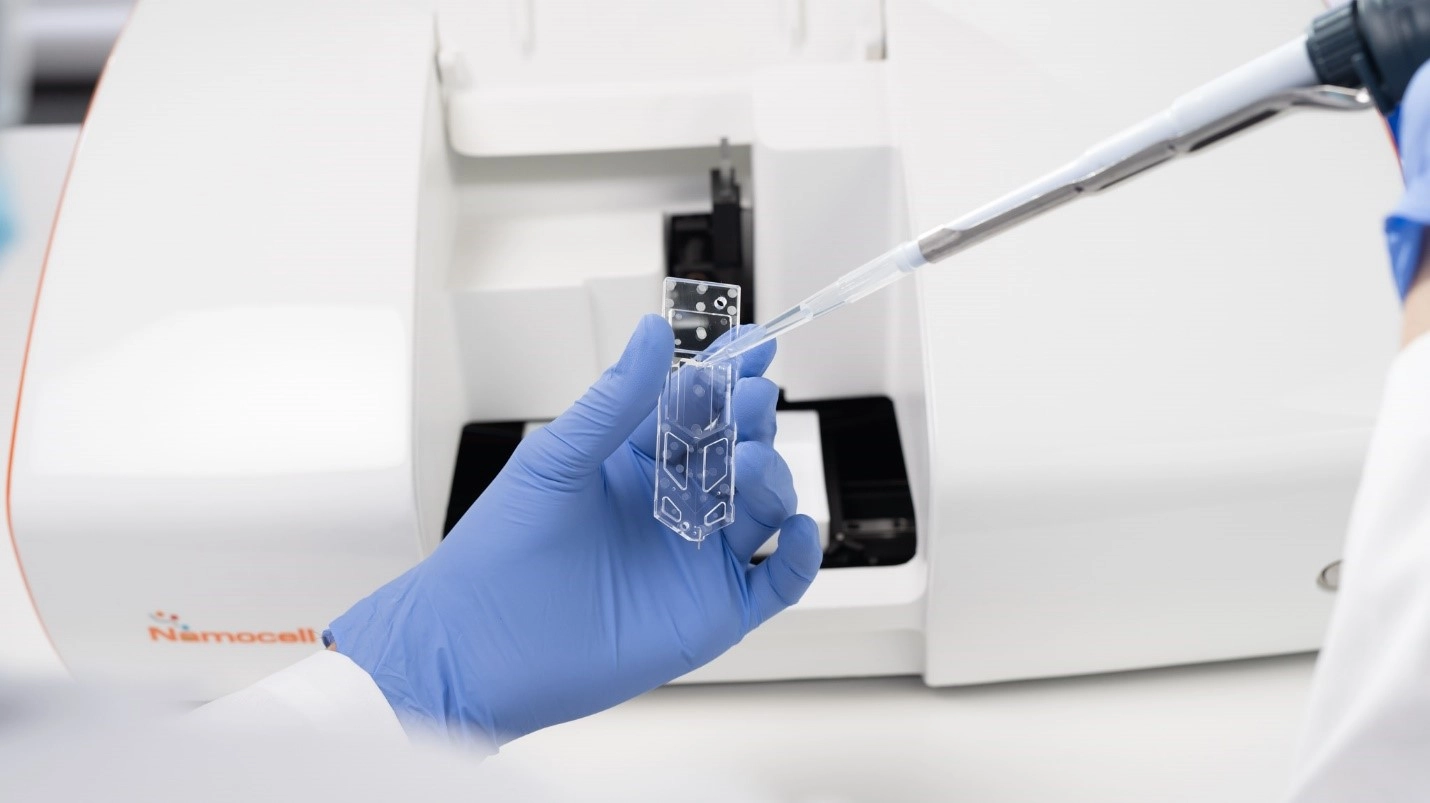 Automated
single-cell dispensers have been designed specifically to address the
challenges mentioned above. By combining microfluidics, flow cytometry, and
liquid dispensing, the Namocell technology sorts cells at a low pressure of less
than 2 psi and deposits cells into a 96-well plate or 384-well plate in 1
minute and 6 minutes, respectively, giving you healthy, viable cells along with
a whole hour back that would otherwise be spent on a FACS sorter.
Automated
single-cell dispensers have been designed specifically to address the
challenges mentioned above. By combining microfluidics, flow cytometry, and
liquid dispensing, the Namocell technology sorts cells at a low pressure of less
than 2 psi and deposits cells into a 96-well plate or 384-well plate in 1
minute and 6 minutes, respectively, giving you healthy, viable cells along with
a whole hour back that would otherwise be spent on a FACS sorter.
The Namocell devices ensure high single-cell deposition efficiency, with over 90% of dispensed wells having a single cell. The design and automation of the Namocell system make the operation simple and intuitive, eliminating the need for specialized personnel or extensive training. The surprisingly small footprint allows for operation of the system entirely inside a standard tissue culture hood, and with disposable cartridges, cross-contamination is not something you need to worry about anymore. Compared to traditional sorters, the Namocell system is much more budget-friendly, making it more accessible to every lab.
To learn more about Namocell and how it has already helped streamline cell development across several labs, visit: https://www.namocell.com/cell-line-development/
References:
1. Hu, P., Zhang, W., Xin, H., & Deng, G. (2016). Single Cell Isolation and Analysis. Frontiers in cell and developmental biology, 4, 116. https://doi.org/10.3389/fcell.2016.00116
2. Ye, M., Wilhelm, M., Gentschev, I., & Szalay, A. (2021). A Modified Limiting Dilution Method for Monoclonal Stable Cell Line Selection Using a Real-Time Fluorescence Imaging System: A Practical Workflow and Advanced Applications. Methods and protocols, 4(1), 16. https://doi.org/10.3390/mps4010016










