Updates on the Frontier of Pharmaceutical and Medicinal Cannabis
The cannabis industry is seeing new opportunities in the areas of research and pharmaceutical development.
Up to this point, a major criticism for the use of cannabis-based medicines by opponents has been the apparent lack of rigorous research and clear understanding about the effects on the body. There has recently been a significant uptick in clinical research, with over 500 active studies on THC and over 300 studies on CBD, as documented in the NIH clinicaltrials.gov site and discussed in a related article. As well, several cannabis-based pharmaceuticals have reached acceptance, including the FDA-approved drug Epidiolex targeted towards select types of seizures and muscle disorders.
What is the Status of Cannabis Research in the US?
Despite this growth, there remain research deficiencies in a range of areas, from the side effects of prolonged treatment to the efficacy of cannabis for chronic diseases such as cancer and others. A major roadblock in the US has involved federal restrictions behind government-sponsored cannabis research. One specific obstacle has been the mandate for use of cannabis supplied only by a single source of research-grade cannabis. Critics within the research community claim that the cannabis available is often insufficient or unique from the types of material that are needed in order to reflect real-world dosages and effects.
This has been an ongoing struggle, and one that has challenged the very notion that medicinal cannabis opponents decry — more research is needed. But how can this happen without the means to obtain the test materials that are required?
How is the Cannabis Research Landscape Changing?
Although the restrictions described above have been in place since the 1960’s, several recent developments may soon open the door to a wide range of cannabis testing material sources. On the legislative front, an announcement back in 2016 stated that a system would be developed and applications for growers to supply research-grade materials would be reviewed. After several years, the DEA is now moving forward with this plan, having identified a number of manufacturers whose applications fit the policy criteria. Recently awarded the Memorandum of Agreement (MOA), several firms have now achieved a crucial step forward towards a working agreement to review cultivation, production, storage, packaging, and distribution. More than 500 licensed researchers wait in the balance of these developments
Better access to more accurate testing materials will support more rigorous and conclusive research. This, in turn, will help unlock the gates towards further pharmaceutical research and discovery.
What Recent Developments Have Arisen in the Cannabis Field?
Many recent research projects have produced interesting results, despite the current restrictions on access.
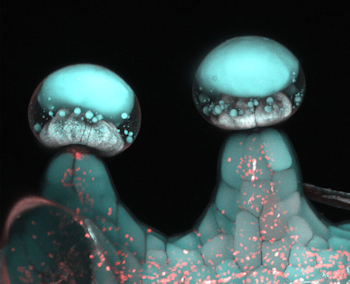 As mentioned above, there are presently a number of clinical studies focused on the major cannabinoids THC and CBD. These two compounds have taken the lion share of the commercial limelight as well. Recent research, however, has started to uncover the relevance of several lesser-abundant, and lesser-known, cannabinoids.
As mentioned above, there are presently a number of clinical studies focused on the major cannabinoids THC and CBD. These two compounds have taken the lion share of the commercial limelight as well. Recent research, however, has started to uncover the relevance of several lesser-abundant, and lesser-known, cannabinoids.
The compound Delta-8 tetrahydrocannabinol (∆-8-THC) has emerged as a less psychoactive analog to THC, with documented analgesic, neuroprotective, and appetite-stimulating properties. It has also recently entered the commercial stage as a gentler version of THC, with less of the psychoactive baggage. What role does ∆-8-THC play and what relevance does it have – in the plant and in the body? Further research aims to find out.
Other compounds such as THC-A, the acid precursor to THC, as well as THC-V and CBN have shown documented physiological effects in the body. Although they are naturally present at much lower concentrations than their relatives, THC and CBD, are the concentrations high enough to elicit their effects? Do these compounds, even at low doses, contribute to the well-documented entourage effect, or the enhancement of bioactivity of cannabis extracts compared with purified THC or CBD? Can the concentrations of such lesser cannabinoids be used to characterize cannabis by identification of genetic, chemical, and environmental traits? Further research is poised to uncover these details and more.
A recent study by an Italian research group introduced the term Phytocannabinomics, or the use of untargeted metabolomics to differentiate between cannabis chemovars or strains. In their work, they looked at 50 known types of Cannabis Sativa, and simultaneously identified 135 phytocannabinoids using untargeted LC-MS. The analysis was able to provide higher resolution differentiation of cannabis chemovars when grown under identical conditions, compared with previous methods which relied solely on the major compounds, THC, CBD, and CBG. Interesting, their method was able to identify, and in some cases reclassify, cannabis chemovars based on the unique composition of the minor cannabinoids. The discovery of several novel cannabis subgroups using this approach may lead to new lines of pharmaceutical investigation.
Outlook
As the landscape changes and further sources of cannabis become available for research, the medicinal cannabis and pharmaceutical fields stand to benefit in many ways. We look forward to the interesting results that flow from this expanding frontier.
Image: Multi‐photon microscopy image of glandular trichome. Credit: Samuels Lab/ University of British Columbia
Visit the Cannabis Laboratory application page for further resources and product listings










