Droplet Digital PCR: Advantages and Applications in Molecular Diagnostics
GEMINI (2025) The evolution of polymerase chain reaction (PCR) technology continues to drive breakthroughs in molecular diagnostics, offering progressively refined tools for quantifying nucleic acids. While quantitative PCR (qPCR) established a high standard for relative measurement, the demand for non-invasive, highly sensitive detection of low-abundance targets, such as circulating tumor DNA (ctDNA), necessitated a technological leap. Droplet Digital PCR (ddPCR) fulfills this need by providing a third-generation methodology that achieves true absolute quantification without reliance on standard curves, fundamentally transforming the capabilities of modern laboratories in fields ranging from oncology to infectious disease surveillance. Droplet Digital PCR (ddPCR) is a revolutionary approach to nucleic acid quantification based on the concept of sample partitioning and endpoint PCR analysis. Unlike traditional qPCR, which relies on monitoring fluorescence during the exponential phase of amplification, ddPCR physically segregates the sample into thousands of tiny, uniform water-in-oil emulsion droplets—typically 20,000 or more per reaction. The reaction mix, containing the template DNA/RNA, primers, and fluorescent probes, is randomly distributed among these droplets. Each droplet acts as an independent micro-reactor. After thermal cycling reaches the endpoint, the droplets are analyzed individually for fluorescence. Droplets containing the target molecule yield a positive signal (fluorescent), while those without the target remain negative (non-fluorescent). The concentration of the target nucleic acid is then calculated based on the ratio of positive to total droplets, utilizing Poisson statistics, delivering absolute quantification with high precision. The scientific power of ddPCR rests entirely on its unique two-step process: partitioning and statistical analysis. This mechanism effectively converts a continuous measurement of concentration into a binary readout, conferring the technology's characteristic robustness and sensitivity. Microfluidic technology is central to creating the uniform, stable droplets that define the digital PCR process. The random distribution of template molecules into these thousands of partitions ensures that most droplets contain either zero or one copy of the target molecule, with a small fraction containing two or more. This separation is key because it isolates the amplification process, making it resistant to minor variations in amplification efficiency or the presence of inhibitors that might affect standard qPCR. Key Analytical Advantages of Digital Partitioning: Elimination of Standard Curves: Concentration is calculated directly from the number of positive partitions, bypassing the need for reference samples or external calibration. Reduced Inhibition Effects: Inhibitors are also partitioned, effectively diluting their concentration to a negligible level within most droplets. Linear Dynamic Range: The measurable concentration range is extensive, extending from single copies of nucleic acid to high concentrations, enabling both rare target detection and high-load quantification. Once the thermal cycling is complete, the droplets are passed sequentially through a detector. The fraction of positive droplets is then used in the Poisson distribution mathematical model to calculate the original template concentration in copies per microliter ( The ability of ddPCR to provide absolute quantification represents its most significant departure from traditional qPCR. While qPCR reports results in terms of In traditional qPCR, the accuracy of quantification is highly dependent on the quality and fidelity of the standard curve used for interpolation. Errors in preparing standards, pipetting variability, or differences in amplification efficiency between the standard and the unknown sample can introduce significant error. ddPCR's independence eliminates these sources of error: Precision and Low Variance: By analyzing tens of thousands of individual events (droplets), the statistical power increases dramatically, leading to significantly lower inter-run and intra-run coefficient of variation (CV). Low Limit of Detection (LOD): The high level of partitioning means that even a few target molecules in the total reaction volume will likely be separated into their own droplet, ensuring they are counted, which is essential for rare target detection. Reference Gene Accuracy: When used for gene expression analysis, ddPCR can quantify target and reference genes (housekeeping genes) independently and absolutely, providing more accurate ratios than the comparative This inherent precision makes ddPCR the gold standard for applications demanding highly accurate, reportable copy counts, such as validating reference materials or determining viral load benchmarks. One of the most transformative applications of ddPCR is in the field of non-invasive molecular diagnostics, particularly liquid biopsy. The technology's enhanced sensitivity is perfectly suited for detecting extremely low-frequency alleles (LFA) and minimal residual disease (MRD) in patient samples. Liquid biopsy relies on detecting circulating tumor DNA (ctDNA) in blood plasma, which often constitutes less than 0.1% of the total circulating cell-free DNA (cfDNA). Detecting these rare targets against a vast background of normal DNA requires a level of sensitivity that qPCR often cannot reliably achieve. ddPCR in Clinical Rare Target Detection: Mutation Monitoring: Tracking therapeutically relevant somatic mutations (e.g., EGFR mutations in lung cancer) and quantifying their change in frequency during treatment. Minimal Residual Disease (MRD): Quantifying residual cancer cells post-treatment, often at levels too low for traditional histology, allowing for earlier detection of relapse. The superior precision of ddPCR allows clinical laboratories to set definitive, reliable thresholds for MRD positivity. Prenatal Diagnostics: Non-invasive prenatal testing (NIPT) for single-gene disorders by accurately quantifying fetal DNA fragments in maternal plasma. The binary readout of ddPCR also inherently reduces background noise, allowing for the clear discrimination between true positive signals from rare targets and instrument or chemical noise, which is a major challenge in highly sensitive qPCR assays. The physical isolation of reactions within the droplets gives ddPCR a high degree of assay robustness, making it notably tolerant of common inhibitors found in complex biological samples compared to bulk PCR methods. Biological sample matrices—such as blood, stool, and environmental samples—often contain substances like hemoglobin, humic acid, and bile salts that can interfere with polymerase enzyme activity. In a bulk qPCR reaction, the presence of these inhibitors in the single reaction volume can lead to delayed Mechanism of Inhibition Tolerance: Concentration Reduction: Inhibitors are distributed across all droplets, effectively diluting the inhibitor concentration within any single droplet, minimizing the impact on the polymerase. Binary Endpoint: Since ddPCR is an endpoint measurement, it does not rely on the kinetics of amplification. Even if the amplification is slightly delayed or slowed by inhibitors in a few droplets, as long as the reaction reaches the endpoint threshold, the droplet is correctly counted as positive. This contrasts sharply with qPCR, where slight delays due to inhibition directly affect the calculated This robustness simplifies sample preparation and reduces the need for extensive purification, accelerating the overall workflow in molecular diagnostics laboratories that routinely handle diverse and challenging sample types. The unique combination of absolute quantification, high sensitivity, and precision has expanded the use of ddPCR far beyond its initial applications in cancer research. It is rapidly becoming a versatile workhorse in several areas of molecular diagnostics and translational science. Application Area Primary Benefit of ddPCR Examples of Targets/Use Oncology Rare Target Detection and Quantification of LFA Quantifying ctDNA, assessing BRAF or KRAS mutation load, MRD monitoring. Infectious Disease Absolute Quantification of Viral/Pathogen Load HIV, Hepatitis B (HBV), Cytomegalovirus (CMV) viral load monitoring, ensuring consistency between labs. Gene Therapy Accurate Quantification of Vector Copies Determining absolute AAV or lentiviral vector copy number per cell (VCN) for safety and dosing studies. Environmental Monitoring High Sensitivity for Low-Level Contaminants Detecting trace levels of genetically modified organisms (GMOs) or waterborne pathogens. Copy Number Variation (CNV) High Precision and Resolution Precise determination of gene copy numbers (e.g., HER2 amplification) without standard curve reliance. These applications illustrate how ddPCR moves clinical and research molecular diagnostics from qualitative or relative reporting into the realm of true digital reporting, providing unambiguous, highly reliable experimental parameters for critical decision-making. The implementation of Droplet Digital PCR offers significant advantages in molecular diagnostics due to its ability to perform true absolute quantification and its superior sensitivity for rare target detection. The reliance on Poisson statistics and digital partitioning ensures high precision and assay robustness against inhibitors, surpassing the limitations inherent in standard qPCR protocols, particularly when analyzing complex or low-concentration biological samples. For laboratories focused on high-stakes clinical monitoring, such as liquid biopsy or viral load quantification, adopting ddPCR technology is crucial for establishing highly reliable and reproducible experimental parameters. What is the key advantage of ddPCR over quantitative PCR (qPCR)? The primary advantage is absolute quantification. ddPCR provides results in discrete copy numbers per volume without relying on a standard curve, leading to higher precision and lower limits of detection, making it superior for rare target detection in molecular diagnostics. Is ddPCR suitable for quantifying all nucleic acid types?
Yes, ddPCR is compatible with DNA, cDNA (for RNA quantification), and cell-free nucleic acids (cfDNA/cfRNA). Its high sensitivity makes it particularly valuable for the low-concentration samples typically analyzed in liquid biopsy applications. How does the Poisson distribution relate to ddPCR accuracy?
The Poisson distribution is the mathematical model used to calculate the original concentration of the template. Because the template is randomly partitioned into droplets, the model accounts for the probability that some droplets received two or more copies, allowing for the highly accurate back-calculation of the true starting concentration, ensuring absolute quantification. Can ddPCR be used for Copy Number Variation (CNV) analysis?
Absolutely. ddPCR offers exceptional resolution for CNV analysis. By quantifying the target gene and a reference gene simultaneously and absolutely, the technology can determine the precise ratio, allowing for the discrimination of small changes in copy number with high precision. This article was created with the assistance of Generative AI and has undergone editorial review before publishing.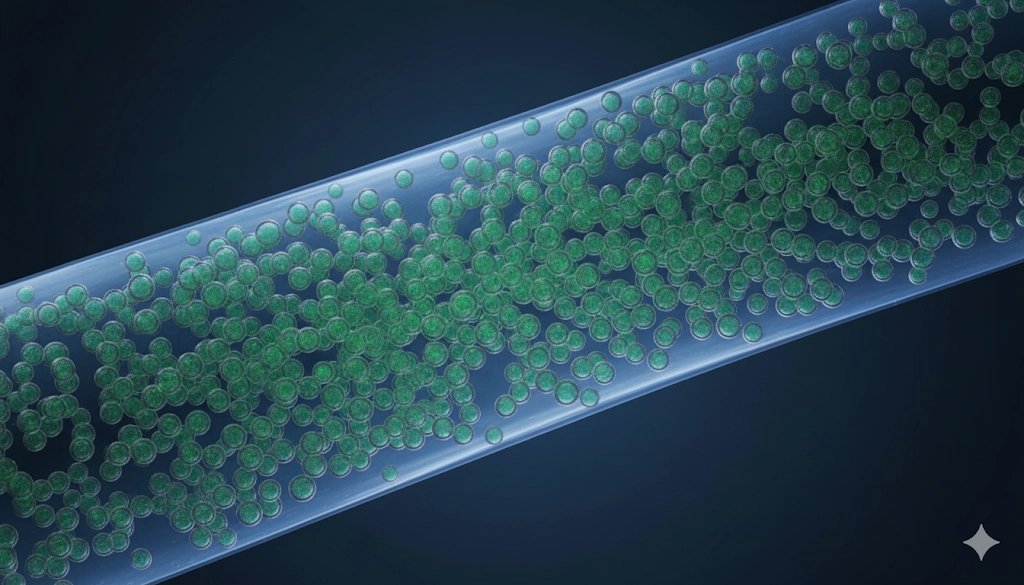
What is Droplet Digital PCR?
Fundamental Mechanism: Partitioning and Poisson Statistics in Digital PCR
The Role of Microfluidics and Partitioning
Unrivaled Precision and Absolute Quantification with ddPCR
The Standard Curve Independence
Enhanced Sensitivity for Rare Target Detection in Liquid Biopsy
Clinical Impact on Oncology and Monitoring
Assay Robustness and Resistance to PCR Inhibition
Overcoming Common Inhibitors in Molecular Diagnostics
Diverse Applications: ddPCR in Clinical Molecular Diagnostics
Key Implementation Areas for ddPCR Technology
Establishing Reliable Absolute Quantification Protocols
Frequently Asked Questions (FAQ) about Droplet Digital PCR










